FIGURE 5.
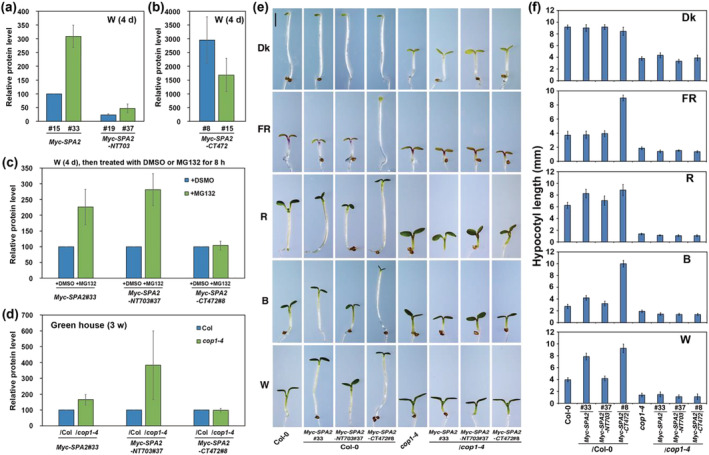
The N‐terminal kinase‐like and coiled‐coil domains of SPA2 are responsible for its stability in light signaling. (a, b) Comparison of relative Myc–SPA2/HSP90, Myc–SPA2–NT703/HSP90, and Myc–SPA2–CT472/HSP90 protein levels in white light. (c) Comparison of relative Myc–SPA2/HSP90, Myc–SPA2–NT703/HSP90, and Myc–SPA2–CT472/HSP90 protein levels after treated with DMSO or MG132. Error bars depict the means ± SD (n = 3). (d) Comparison of relative Myc–SPA2/HSP90, Myc–SPA2–NT703/HSP90, and Myc–SPA2–CT472/HSP90 in wild type (Col‐0) or cop1‐4 mutant background. (e) Morphology of Myc–SPA2 (line 33), Myc–SPA2–NT703 (line 37), and Myc–SPA2–CT472 (line 8) in both the WT (Col‐0) and cop1‐4 mutant backgrounds. Seedlings were grown in the dark (Dk) or under FR (1.9 μmol·m−2·s−1), R (24.4 μmol·m−2·s−1), B (11.6 μmol·m−2·s−1), or W (7.8 μmol·m−2·s−1) for 4 days. Bar = 1 mm. (f) Quantification of hypocotyl lengths of Myc–SPA2 (line 33), Myc–SPA2–NT703 (line 37), and Myc–SPA2–CT472 (line 8) in both the WT (Col‐0) and cop1‐4 mutant backgrounds. See also Figures S7 and S8
