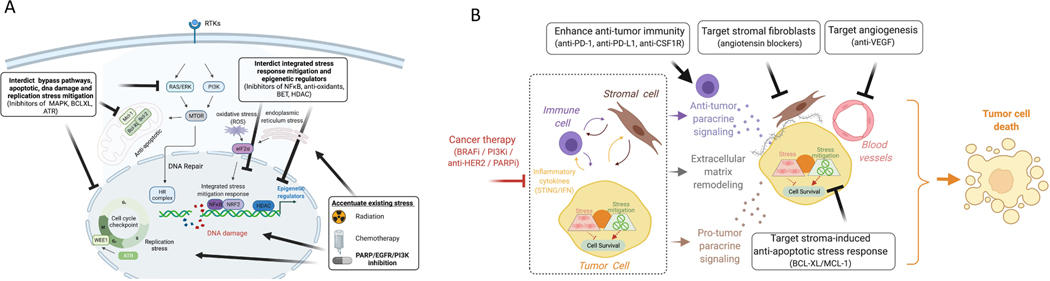
Figure 3. Strategies to induce tumor cell death by exploiting stress adaptation, accentuating existing stress and targeting emergent vulnerabilities in the tumor ecosystem.
(A) Example of therapeutic opportunities emerging from therapy-induced stress. PARP inhibitors induce DNA damage and replication stress that results in cell cycle arrest and engagement of DNA repair pathways. This diagram show strategies involving combination therapies to enhance the efficacy of PARP inhibition that increase stress via radiation or chemotherapy. In addition, there are multiple approaches to interdict the stress mitigation process via 1) blocking anti-apoptotic survival proteins such as BCL-XL, 2) inhibiting upstream mediators of DNA repair (RAS/ERK inhibition) or cell cycle checkpoints (ATR), 3) blocking epigenetic reprograming (HDAC/BET) and 4) stress mitigation programs (e.g. anti-oxidant NRF2, NFkB). (B) Targeting emergent opportunities in the tumor ecosystem represents another approach to develop rational combination therapies. Tumor cells in therapy-resistant tumor ecosystems can be targeted by 1) inhibiting immune checkpoints to augment anti-tumor immunity that is mediated via tumor-immune paracrine signaling, 2) blocking therapy-induced extracellular matrix remodeling that enhances recruitment of cytotoxic immune cells, 3) targeting anti-apoptotic signaling that is induced via stromal-derived cytokines and extracellular matrix remodeling, and 4) blocking angiogenesis to normalize blood vessels and enhance immune cell recruitment.