Figure 1. The CDK4 and CDK6 pathway in cancer.
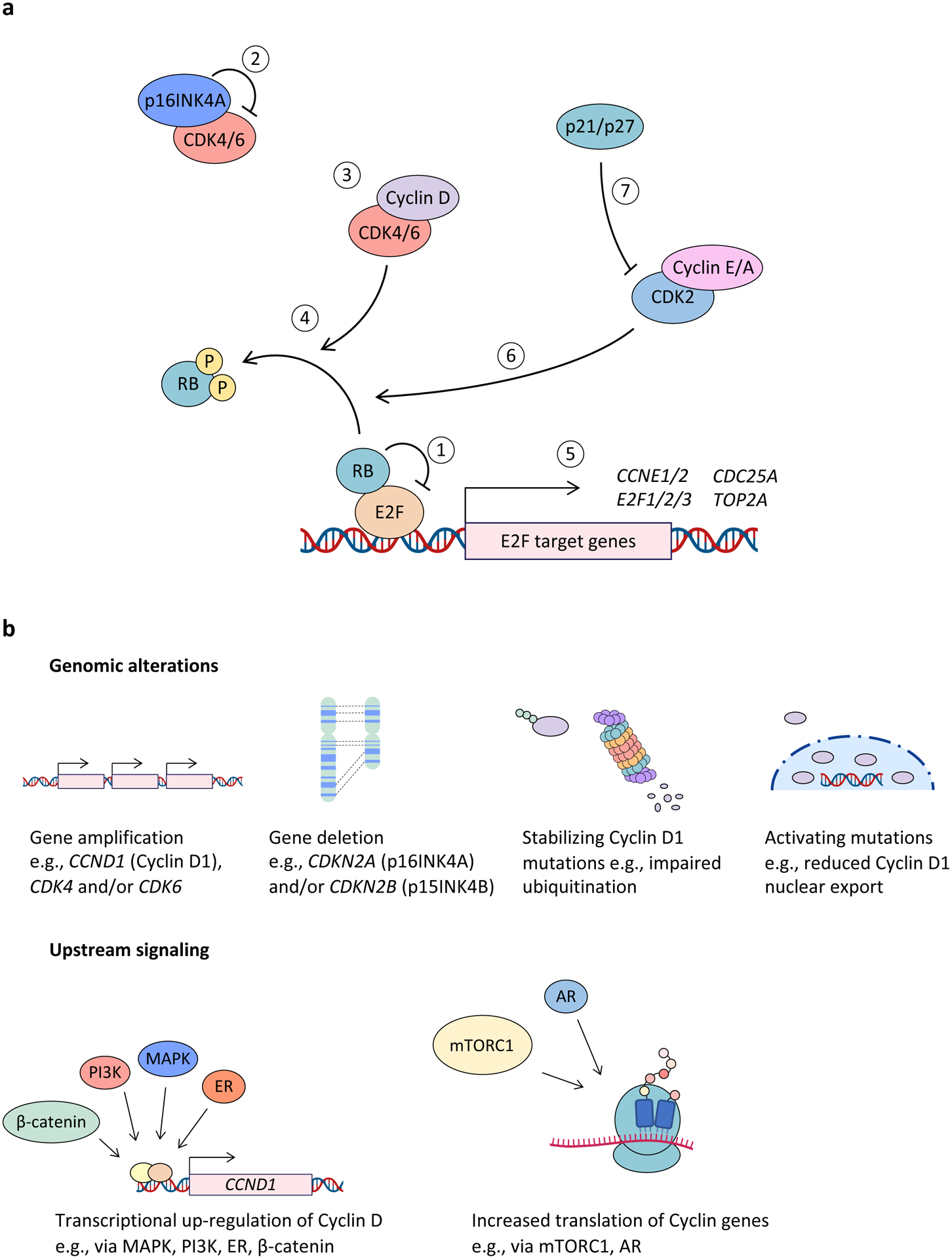
a, Hypophosphorylated RB binds to and represses E2F family transcription factors (1). Negative regulation of cyclin-dependent kinase 4 (CDK4) and CDK6 (CDK4/6) activity is mediated primarily by the INK4 family of cell cycle inhibitors (INK4B (p15; encoded by cyclin-dependent kinase inhibitor 2B (CDKN2B)), INK4A (p16; encoded by CDKN2A), INK4C (p18), and INK4D (p19)), which bind to monomeric CDK4 and CDK6 to form inactive binary complexes (2). Mitogen or growth factor stimulation drives cyclin D up-regulation, leading to CDK4/6 activation (3). RB phosphorylation by cyclin D–CDK4/6 complexes promotes dissociation of RB–E2F binding (4). This in turn allows for E2F-mediated expression of genes required for cell cycle progression (5), which leads to progression through G1 phase and into S-phase. RB phosphorylation by cyclin E–CDK2 and cyclin A–CDK2 complexes (6) also promotes RB–E2F dissociation to drive progression into S phase. On the other hand, WAF1 and KIP family proteins, such as p21 (WAF1), p27 (KIP1) and p57 (KIP2), inhibit CDK2 and are important for inducing cell cycle arrest (7). Of note, p21 and p27 (p21/p27) have been shown to inhibit CDK4/6 activity in some instances and in other instances to stabilize cyclin D–CDK4/6 and thereby form an active trimeric holoenzyme. b, Major mechanisms responsible for dysregulated CDK4/6 activity in cancer include genomic alterations as well as activation of upstream signalling pathways that may up-regulate this pathway at the transcriptional, translational and post-translational levels. AR, androgen receptor; CCN, cyclin; ER, oestrogen receptor; mTORC1, mTOR complex 1.
