Figure 2. A conceptual framework to understand the effects of CDK4 and CDK6 inhibitors in cancer.
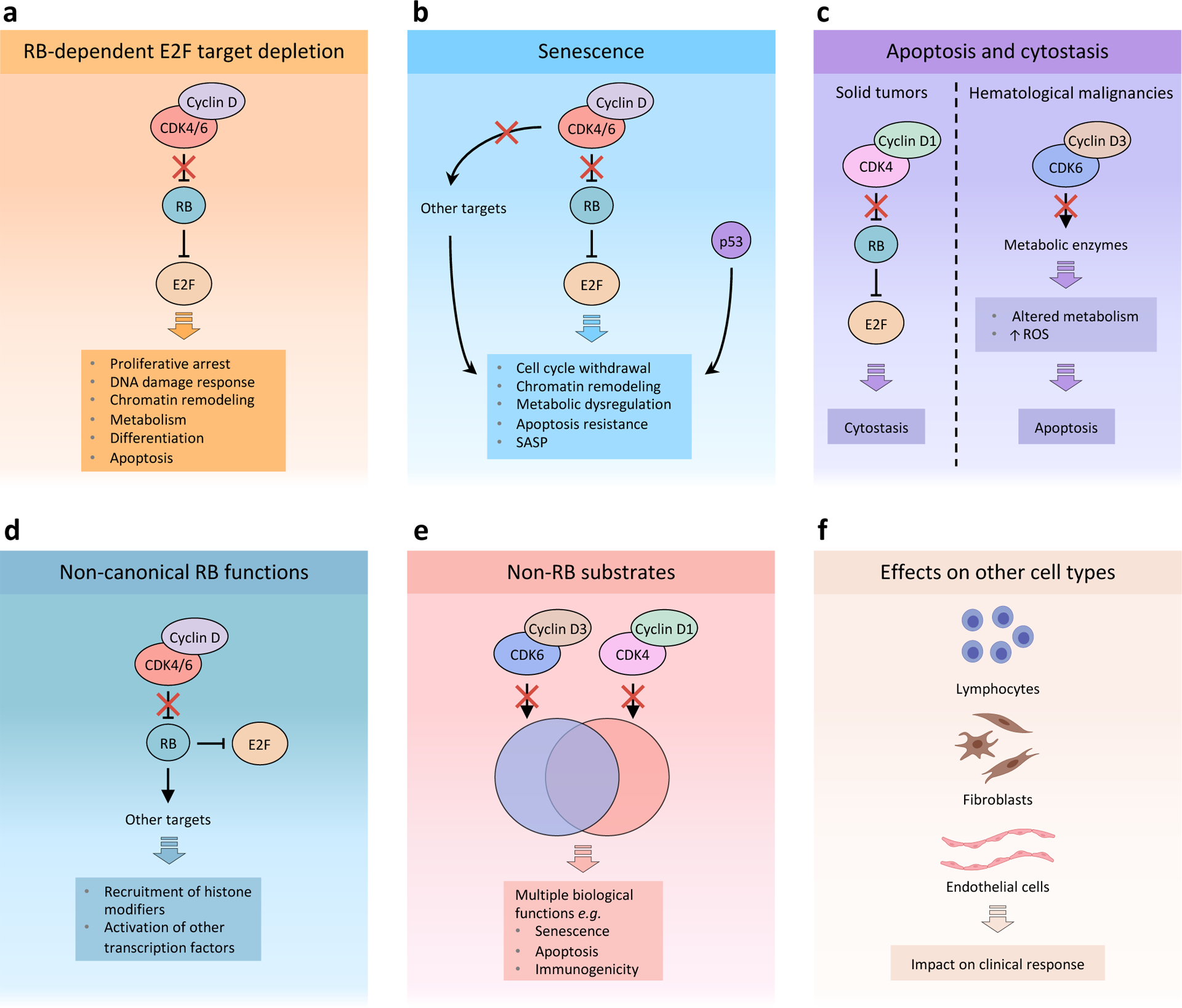
Response to cyclin-dependent kinase 4 (CDK4) and CDK6 (CDK4/6) inhibitors depends on multiple factors that affect the final outcome, including context-dependent tumour cell-intrinsic and -extrinsic features. a, RB-dependent E2F target depletion: Blockade of RB phosphorylation by cyclin D–CDK4/6 complexes leads to sustained binding to and inhibition of E2F by RB. A major consequence of E2F inactivation by RB is proliferative arrest. Moreover, E2F functions in various additional processes that are affected by CDK4/6 inhibition, including DNA damage response, chromatin remodelling, metabolism, differentiation and apoptosis. b, Senescence: CDK4/6 inhibitors may induce an RB-dependent senescent phenotype characterized by cell cycle withdrawal, chromatin remodelling, metabolic dysregulation, resistance to apoptosis and a senescence-associated secretory phenotype (SASP). Downregulation of other CDK4/6 targets, such as forkhead box protein M1 (FOXM1) and DNA methyltransferase 1 (DNMT1), is thought to enhance the senescent phenotype. In addition, p53 plays distinct and overlapping roles with RB in inducing senescence. Current evidence suggests that loss of p53 function may significantly affect senescence induced by CDK4/6 inhibitors, although additional studies have shown that CDK4/6 inactivation can still induce senescent phenotypes in the absence of wild type p53. c, Apoptosis and cytostasis: Induction of apoptosis or cytostasis appears to be cell type-dependent and may depend on differences in signalling between CDK4 and CDK6. In haematological malignancies, inhibition of CDK6–cyclin D3 complexes may lead to an RB-independent state of metabolic dysregulation that results in apoptosis. On the other hand, solid tumours such as breast cancer often depend primarily on CDK4–cyclin D1 activity for proliferation. In this case, CDK4/6 monotherapy predominantly induces RB-dependent proliferative arrest as a result of sustained E2F inhibition. d, Non-canonical RB functions: In addition to inhibiting E2F, RB has been shown to exert other functions, such as mediating chromatin remodelling and activation of other transcription factors. e, Non-RB substrates: In addition to phosphorylating and inhibiting RB, CDK4 and CDK6 phosphorylate a set of unique and overlapping targets that play important functions in numerous biological processes, including senescence, apoptosis and immunogenicity. f, CDK4/6 inhibitors have been shown to exert direct effects on multiple cell types normally present within the tumour microenvironment, including lymphocytes, fibroblasts and endothelial cells. Several studies have shown CDK4/6 inhibitors can directly and indirectly promote anti-tumour T lymphocyte effector function and inhibit immunosuppressive regulatory T (Treg) cells. CDK4/6 inhibitors also induce senescence phenotypes in fibroblasts, which could potentially impair response to therapy by promoting tumour growth, and cell cycle arrest in endothelial cells, which has the potential to impact on clinical response. ROS, reactive oxygen species.
