Table 1.
CDK4 and CDK6 inhibitors approved or under clinical development.
| Agent | Company | Selectivity (IC50) | Clinical development |
|---|---|---|---|
| Approved | |||
Palbociclib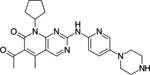 |
Pfizer | CDK4: 11 nM CDK6: 16 nM |
Approved for HR+, HER2− advanced breast cancer in combination with hormonal therapy |
| Abemaciclib |
Eli Lilly | CDK4: 2 nM CDK6: 10 nM |
Approved for HR+, HER2− advanced breast cancer in combination with hormonal therapy Approved as monotherapy for advanced HR+, HER2− breast cancer Approved as adjuvant therapy for high-risk, early-stage HR+, HER2− breast cancer in combination with hormonal therapy |
Ribociclib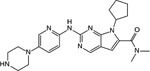 |
Novartis | CDK4: 10 nM CDK6: 39 nM |
Approved for HR+, HER2− advanced breast cancer in combination with hormonal therapy |
Trilaciclib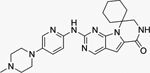
|
G1 Therapeutics | CDK4: 1 nM CDK6:4 nM |
Approved to reduce chemotherapy-induced bone marrow suppression in patients with extensive-stage SCLC. |
| Phase III | |||
| Dalpiciclib (SHR6390) | Jiangsu Hengrui Medicine | CDK4: 12 nM CDK6: 10 nM |
Phase III for HR+, HER2− breast cancer in combination with hormonal therapy; phase I/II for multiple tumor types in combination with hormone, targeted or immune therapy |
| Phase II | |||
| PF-06873600 | Pfizer | CDK2: 0.09 nM (Ki) CDK4: 0.13 nM (Ki) CDK6: 0.16 nM (Ki) |
Phase II for HR+, HER2− metastatic breast cancer, TNBC and gynecological cancers in combination with hormonal therapy |
| Phase I/II | |||
| Lerociclib (G1T38) | G1 Therapeutics | CDK4: 1 nM CDK6: 2 nM CDK9: 28 nM |
Phase I/II for HR+, HER2− metastatic breast cancer in combination with fulvestrant (SERD); phase I/II for EGFR-mutant metastatic NSCLC in combination with osimertinib (EGFR inhibitor) |
| Birociclib (XZP-3287) | Jilin Sihuan Pharmaceutical/Xuanzhu Pharma | Not available | Phase I/II for HR+, HER2− advanced breast cancer |
| BPI-1178 | Beta Pharma | Not available | Phase I/II for advanced solid tumors, and for HR+, HER2− advanced breast cancer in combination with hormone therapy |
| FCN-437C | Fochon Pharmaceuticals | Not available | Phase I/II for HR+, HER2− advanced breast cancer in combination with letrozole (aromatase inhibitor) |
| TQB3616 | Chia Tai Pharmaceutical Group | Not available | Phase I/II for HR+, HER2− advanced breast cancer in combination with fulvestrant, and for advanced lung cancer in combination with anlotinib (VEGFR inhibitor) or standard chemotherapy |
| Phase I | |||
| AMG-925 (FLX925) | Amgen | CDK4: 1.5 nM CDK6: 8 nM FLT3: 2.4 nM |
Phase I/Ib for relapsed or refractory AML |
| R547 | Hoffmann-La Roche | CDK1: 2 nM (Ki) CDK2: 3 nM (Ki) CDK4:1 nM (Ki) |
Phase I for advanced solid cancers |
| BPI-16350 | Betta Pharmaceuticals | Not available | Phase I for advanced solid cancers |
| CS3002 | CStone Pharmaceuticals | Not available | Phase I for advanced solid cancers |
| HS-10342 | Jiangsu Hansoh Pharmaceutical | Not available | Phase I for advanced solid cancers |
| PF-06842874 | Pfizer | Not available | Phase I in healthy participants |
| TY-302 | TYK Medicines | Not available | Phase I for advanced solid cancers, and for HR+, HER2− breast advanced cancer in combination with tamoxifen |
AML, acute myeloid leukaemia; CDK, cyclin-dependent kinase; EGFR, epidermal growth factor receptor; FLT3, FMS-like tyrosine kinase 3; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; SCLC, small-cell lung cancer; SERD, selective estrogen receptor degrader; TNBC, triple-negative breast cancer; VEGFR, vascular endothelial growth factor receptor.
