Abstract
Tepotinib, the novel MET-tyrosine kinase inhibitor, shows an antitumor effect for patients with non-small-cell lung cancer (NSCLC) harboring MET exon 14 skipping mutation. In January 2022, the AmoyDx® Pan Lung Cancer polymerase chain reaction Panel (AmoyDx® panel), which had a shorter turnaround time than the conventional test, was launched in Japan as a tepotinib companion test. We report a patient with an advanced MET-mutant NSCLC promptly diagnosed using the AmoyDx® panel and successfully treated with tepotinib. Although the patient's performance status (PS) worsened due to the rapid tumor progression and lung abscess formation, the tumor shrank immediately after tepotinib treatment with marked PS improvement.
Keywords: MET, Tepotinib, Non-small-cell lung cancer
Introduction
MET exon 14 skipping mutations are present in approximately 3% of non-small-cell lung cancers (NSCLCs) [1]. In 2020, two MET-tyrosine kinase inhibitors, tepotinib and capmatinib, were approved for the treatment of NSCLC, harboring MET exon 14 skipping mutations. These drugs have yielded a good progression-free survival of 6–12 months in clinical trials [2, 3]. The approval of tepotinib and capmatinib was accompanied by the ArcherMET and FoundationOne® CDx tests, respectively. However, these kits had a long turnaround time (TAT) (ArcherMET: 9–12 business days, FoundationOne® CDx: 14 business days), which may cause delayed treatment initiation. The AmoyDx® Pan Lung Cancer polymerase chain reaction (PCR) Panel, which had a short TAT (3 business days), was approved in 2021 and launched in Japan in January 2022. Although tepotinib achieved a favorable progression-free survival for patients with a good performance status (PS) (such as 0–1) in the VISION trial, its efficacy for patients with a poor PS has not been established [2]. We report a patient with advanced NSCLC, harboring a MET exon 14 skipping mutation, successfully and promptly treated with tepotinib as the first-line therapy. The tumor was diagnosed as an NSCLC, harboring a MET exon 14 skipping mutation, using the AmoyDx® Pan Lung Cancer PCR Panel within 1 week. The patient had a poor PS due to the rapid tumor progression and lung abscess formation. Therefore, he was treated with tepotinib to prevent severe infection induced by cytotoxic chemotherapy.
Case Presentation
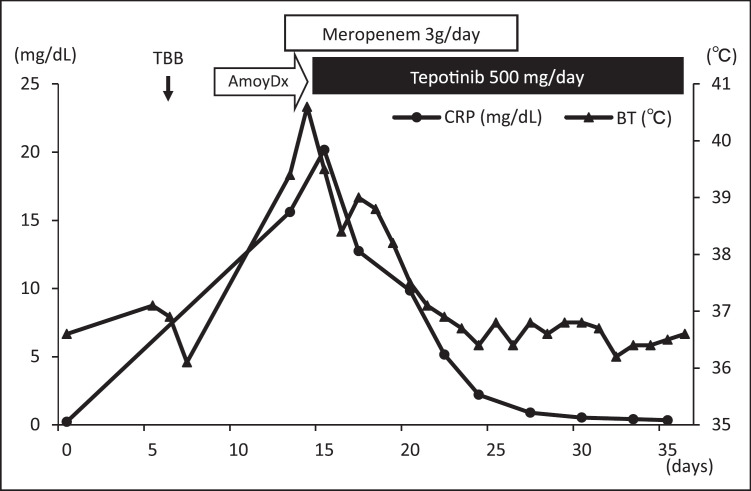
A 65-year-old man complained of dyspnea for 1 month and was referred to our hospital for the evaluation of an abnormal lung shadow. On arrival, he had a PS of 1. The chest computed tomography (CT) scan showed a lung tumor in the right middle lobe (Fig. 1a). Positron emission tomography showed fluorodeoxyglucose uptake in the lung tumor and bone. Six days after his initial arrival, a transbronchial biopsy of the lung tumor was performed. The rapid on-site evaluation was positive, and histology revealed adenocarcinoma 3 days after bronchoscopy. Four days after bronchoscopy, he developed a spiking fever at home, accompanied by right chest pain and yellow sputum production. Three days later, the patient returned to our hospital. Rapid lung cancer growth and multiple bone metastases were detected on CT imaging (Fig. 1b). The patient was diagnosed with rapidly progressive lung cancer and a lung abscess. He was treated with meropenem (3 g/day). Three days later, his general condition did not improve, and his PS declined to 3 due to high fever. At that time, the fresh frozen tissue specimens and formalin-fixed paraffin-embedded samples, which were submitted to the Cancer Genome Screening Project for Individualized Medicine in Japan 6 days earlier, revealed a MET exon 14 skipping mutation based on the AmoyDx® Pan Lung Cancer PCR Panel. Tepotinib treatment was initiated 16 days after the patient's first arrival at our hospital. His fever gradually improved, and chest CT documented tumor shrinkage. Thus, the patient's general condition improved and reached a PS of 0 (Fig. 1c, d, 2). Although he experienced mild leg edema, he showed no severe adverse effects related to tepotinib, and the treatment was continued for more than 2 months. The tumor was also evaluated using ArcherMET, which yielded a positive result 43 days after the patient's first arrival at our hospital.
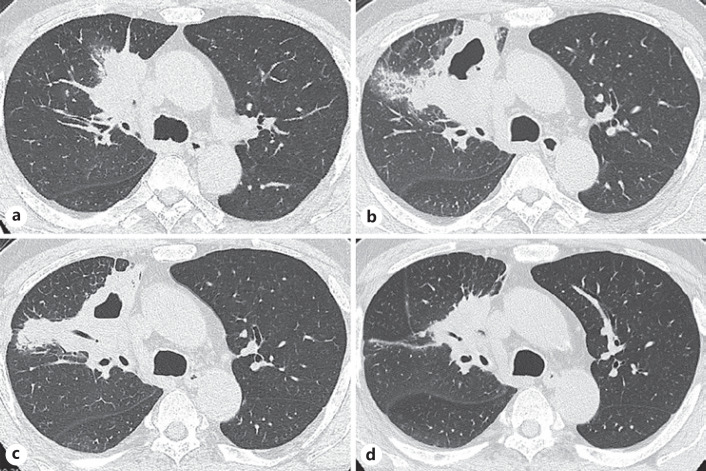
Fig. 1.
Chest CT image before and after the tepotinib treatment. a The chest CT image on the patient's first arrival at our hospital. b The lung tumor grew rapidly 13 days after his first arrival. c, d The lung tumor shrank after tepotinib treatment. Twelve days after tepotinib treatment (c) and 1 month after tepotinib treatment (d).
Fig. 2.
The patient's clinical course before and after the tepotinib treatment.
Discussion
We report a case of NSCLC combined with a lung abscess, harboring a MET exon 14 skipping mutation, which was successfully and promptly treated with tepotinib. This was the first successfully treated case of concomitant lung cancer and abscess with tepotinib as the first-line treatment. This case is clinically suggestive for two reasons. First, the MET exon 14 skipping mutation was diagnosed using the newly approved AmoyDx® Pan Lung Cancer PCR Panel within 6 days, and the patient was treated with tepotinib within a week from his diagnosis. Second, although the patient had a poor PS due to lung cancer progression and lung abscess formation, he was successfully treated with tepotinib, and his PS improved significantly.
The patient was diagnosed with NSCLC, harboring the MET exon 14 skipping mutation, using the newly approved AmoyDx® Pan Lung Cancer PCR Panel with a short TAT. The MET exon 14 skipping mutation is a rare mutation, accounting for approximately 3% of NSCLC. ArcherMET, the typical companion test for tepotinib, takes more than 2 weeks from histological diagnosis to the initiation of the first-line treatment with MET-tyrosine kinase inhibitor. However, in this case, the newly approved diagnostic test AmoyDx® Pan Lung Cancer PCR Panel was used, resulting in prompt diagnosis and treatment. Achieving a short TAT of less than 1 week, this companion test was approved in 2021 and launched in Japan in January 2022.
Second, the prompt access to tepotinib achieved a favorable antitumor effect and dramatically improved the patient's PS. Alterations in MET were correlated with poor survival among patients with wild-type NSCLC [4]. In this case, the primary lung tumor and bone metastasis grew rapidly. Moreover, a lung abscess with a cavity was formed. Therefore, treating the patient required controlling both the tumor growth and infection. Although conventional platinum-based cytotoxic chemotherapy is the standard treatment for advanced lung adenocarcinoma, it causes myelosuppression, which results in severe and fatal infections. The MET skipping mutation was identified within 1 week from the histological diagnosis in this case. Therefore, tepotinib was immediately obtained, and a favorable antitumor effect was achieved. According to the VISON phase II trial, patients harboring the MET exon 14 skipping mutation with good PS (0–1) were eligible for receiving tepotinib treatment [2]. However, its efficacy for patients with poor PS has not been established. This study showed that tepotinib is a viable treatment choice for patients with poor PS due to tumor growth and lung abscess formation.
Conclusion
We encountered a rare case of NSCLC, combined with lung abscess formation, harboring a MET exon 14 skipping mutation, which was successfully and promptly treated with tepotinib. Rapid tumor growth and lung abscess formation result in a poor PS. However, this case showed that tepotinib is a viable treatment option that prevented severe infection.
Statement of Ethics
This case report was prepared and completed in accordance with the guidelines of the 2013 revised Helsinki Declaration. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. This retrospective review of patient data did not require ethical approval in accordance with local/national guidelines.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Go Makimoto searched the literature and contributed to the care of the patient and to the writing of the final draft. Atsushi Shimonishi, Kadoaki Ohashi, Kiichiro Ninomiya, Hisao Higo, Yuka Kato, Masanori Fujii, Toshio Kubo, Eiki Ichihara, Katsuyuki Hotta, and Masahiro Tabata have contributed to the care of the patient and to the editing of the manuscript. Yoshinobu Maeda and Katsuyuki Kiura have contributed to the scientific review and have supervised the patient treatment. All the authors have made significant contributions to the manuscript and have read and approved the final manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the patient for providing consent to publish clinical information and data. We also thank Editage (www.editage.jp) for English language editing of this manuscript.
References
- 1.Reungwetwattana T, Liang Y, Zhu V, Ou SI. The race to target MET exon 14 skipping alterations in non-small cell lung cancer: the why, the how, the who, the unknown, and the inevitable. Lung Cancer. 2017 Jan;103:27–37. doi: 10.1016/j.lungcan.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020 Sep 3;383((10)):931–43. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020 Sep 3;383((10)):944–57. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
- 4.Wu YL, Soo RA, Locatelli G, Stammberger U, Scagliotti G, Park K. Does c-Met remain a rational target for therapy in patients with EGFR TKI-resistant non-small cell lung cancer? Cancer Treat Rev. 2017 Dec;61:70–81. doi: 10.1016/j.ctrv.2017.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.