Abstract
Although mRNA vaccine responses following previous coronavirus disease 2019 (COVID-19) infection have not been assessed in trials, it has been shown that serological evidence of previous COVID-19 generates strong humoral and cellular responses to one dose of mRNA vaccine. We describe a patient with prior COVID-19 infection who developed acute transient encephalopathy with elevated inflammatory markers within 24 h of her first injection of Moderna COVID-19 vaccine. A 69-year-old cognitively normal woman presented with intermittent inattention, disorientation, left/right confusion, weakness, gait instability, and decreased speech. Head CT, brain MRI and MRA, complete blood count, liver enzymes, hepatitis B serology, ammonia, thyroid function, vitamin B12, and pulse oximetry were normal. Electroencephalography performed 48 h after symptom onset showed diffuse triphasic waves, diffuse theta and delta slowing, and no posterior dominant rhythm. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG was positive and inflammatory markers were elevated. On day 5 post-vaccine, she returned to her baseline, without neurological sequelae. The reported patient likely developed a transient inflammatory encephalopathy associated with an abnormal immunologic reaction to one dose of COVID-19 vaccine, in the setting of remote COVID-19 infection (1 year prior), SARS-CoV-2 IgG-positivity, and multiple comorbidities. Physicians should be alert to possible postvaccination reactogenicity in individuals with SARS-CoV-2 IgG-positivity, including risk of neuro-inflammation.
Keywords: COVID-19 vaccine, Vaccine adverse events, Encephalopathy, SARS-CoV-2
Introduction
Recent studies report that individuals with previous coronavirus disease 2019 (COVID-19) infection and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG-positivity have higher antibody titer responses to one dose of mRNA vaccine than those not previously infected [1]. As serological evidence of previous COVID-19 generates strong humoral and cellular responses to one dose of mRNA vaccine, it is therefore feasible that a mutated/abnormal immunological reaction could cause unexpected and rare adverse events following vaccination (AEFV) [2]. We describe a patient with prior COVID-19 infection and SARS-CoV-2 IgG-positivity, who developed acute transient encephalopathy with elevated inflammatory markers after her first Moderna (Cambridge, MA) COVID-19 vaccine injection.
Case Report/Case Presentation
A 69-year-old cognitively normal woman developed confusion, weakness, gait instability, and decreased speech within 24 h of her first Moderna COVID-19 vaccine injection. Comorbidities included well-controlled hypertension, end-stage renal disease on hemodialysis, diabetes, atrial fibrillation, and COVID-19 infection (March 2020, 12 months before the vaccine injection). She presented to the emergency room on day 3 post-vaccine for persisting symptoms and skipped dialysis. Examination showed fluctuating attention, disorientation to time and space, left/right confusion, and intermittently followed one-step commands. Naming, reading, repetition were intact; however, she was nonfluent and intermittently incoherent. Neurological examination was otherwise nonfocal. At admission, head CT, complete blood count, liver enzymes, hepatitis B serology, ammonia, thyroid function, vitamin B12, and pulse oximetry were normal, SARS-CoV-2 RNA negative. Renal function was at her baseline (Table 1). Home medications, including anticoagulation, statin, insulin, and phosphate binder, were continued, and patient underwent hemodialysis. Electroencephalography showed diffuse triphasic waves, diffuse theta and delta slowing, and no posterior dominant rhythm.
Table 1.
Daily laboratory tests during admission
| Blood tests (normal range) | Hospital day 1 post-vaccine day 3ϕ | Hospital day 2* post-vaccine day 4 | Hospital day 3 post-vaccine day 5* | Hospital day 4 post-vaccine day 6ϕ | Hospital day 5 post-vaccine day 7 |
|---|---|---|---|---|---|
| WBC (3.5–10 K/µL) | 6.02 | 5.50 | 6.5 | 4.88 | 4.06 |
| Hb (12–16 g/dL) | 13.3 | 13.6 | 12.5 | 13.1 | 12.9 |
| PLT (130–400 K/µL) | 108 | 115 | 120 | 124 | 153 |
| Glucose (70–99 mg/dL) | 120 | 151 | 128 | 111 | 152 |
| BUN (7–25 mg/dL) | 47 | 33 | 42 | 35 | 38 |
| Creatinine (0.6–1.3 mg/dL) | 7.8 | 6.4 | 6.9 | 6.9 | 5.1 |
| GFR_AA (≥60 mL/min/1.73 min) | 6 | 8 | 7 | 7 | 10 |
| Albumin (3.5–5.7 g/dL) | 3.8 | − | 3.5 | 3.5 | 3.4 |
| ALP (34–104 U/L) | 127 | − | 107 | 102 | 98 |
| AST (13–39 U/L) | 14 | − | 17 | 14 | 11 |
| ALT (7–52 U/L) | 9 | − | 9 | 12 | 10 |
| Ammonia (18–72 µmol/L) | − | − | 24 | − | − |
| Calcium (8.2–10 mg/dL) | − | 9.2 | 8.7 | 9.3 | 8.8 |
| Magnesium (1.9–2.7 mg/dL) | − | 2.1 | 2.1 | 2.3 | − |
| Phosphorus (2.5–5 mg/dL) | − | 4.3 | 4.5 | 4.9 | − |
| Sodium (136–145 mmol/L) | 134 | 136 | 135 | 142 | 137 |
| Potassium (3.5–5.1 mmol/L) | 5.4 | 4.4 | 4.6 | 4.9 | 3.3 |
| TSH (0.38–4.7 µIU/mL) | 0.91 | 0.91 | − | − | − |
| ESR (0–30 mm/h) | − | − | 12 | 110 | − |
| CRP (0–8 mg/dL) | − | − | 82 | 108 | − |
| Blood culture | − | − | 2 sets no growth | − | − |
| Sputum culture | − | − | Negative | − | − |
| SARS-CoV-2 RNA | Negative | − | Negative | − | − |
WBC, white blood count; Hb, hemoglobin; PLT, platelets; BUN, blood urea nitrogen; GFR_AA, glomerular filtration rate, African American; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine transaminase; TSH, thyroid stimulating hormone; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Hemodialysis performed in the afternoon.
After febrile episode.
On day 4 post-vaccine (day 2 of hospitalization), her temperature increased to 100.6 F (baseline 97.8–98.2); infectious evaluation (complete blood count, chest X-ray, blood, sputum and Legionella cultures, and SARS-CoV-2 RNA) was negative. SARS-CoV-2 IgG was positive, and inflammatory markers were elevated and up-trending (C-reactive protein from 82 to 207 [normal 0–8 mg/L], erythrocyte sedimentation rate from 12 to 110 [normal 0–30 mm/h]). Given the isolated febrile episode and the strict temporal correlation between symptom onset and the Moderna COVID-19 vaccine injection, a joint decision with infectious disease team was made to defer central nervous system (CNS) antibiotic and antiviral coverage after performing a lumbar puncture and an MRI of the brain.
On day 5 post-vaccine (day 3 of hospitalization), patient's symptoms suddenly and completely resolved, brain MRI and MRA showed no acute findings, and lumbar puncture procedure was cancelled. She was monitored for another day in the hospital and discharged at her baseline.
Discussion/Conclusion
We report a patient who developed intermittent inattention, disorientation to time and space, left/right confusion, decreased fluency, generalized weakness, and gait instability within 24 h of her first Moderna COVID-19 vaccine injection. The presentation was highly suspicious for toxic-metabolic encephalopathy or delirium, given the presence of inattention and fluctuating symptoms. Extensive work-up was performed and metabolic disturbances, new onset of medical conditions, substance intoxication or withdrawal were ruled out. MRI brain did not show any acute ischemic stroke; the absence of focality and the sudden return at baseline on day 5 post-vaccine (day 3 of hospitalization) made the possibility of a DWI-negative stroke unlikely. Our patient's symptomatology was highly suspicious for CNS infection, and a CSF study would have been ideal to rule out bacterial meningitis or viral encephalitis. However, given the isolated febrile episode, the sudden and complete resolution of patient's symptoms on day 5 post-vaccine without any treatment, and a negative systemic infectious work-up (including 2 sets of blood cultures, sputum analysis, and 2 SARS-CoV-2 RNA [at admission and after the febrile episode]), we felt confident that a CNS infectious process was highly unlikely, even in the absence of CSF analysis. Given the very strict temporal correlation between symptom onset and the Moderna COVID-19 vaccine injection, and the extensive negative work-up, we suggest that an exaggerated immune response to mRNA vaccine in the setting of remote COVID-19 infection (1 year prior) is the most likely cause of the transient inflammatory encephalopathy. This abnormal response might have been amplified by our patient's systemic persistent inflammation, a common feature of end-stage kidney disease and hemodialysis [3]. Positive SARS-CoV-2 IgG antibodies show persistent immune response after COVID-19 infection. Although mRNA vaccine responses following previous COVID-19 infection have not been assessed in trials, serological evidence of previous COVID-19 generates strong humoral and cellular responses to one dose of mRNA vaccine [2]. While it is unclear if prior COVID-19 infection played a role in our patient's transient inflammatory encephalopathy, we believe that the known exaggerated immune response to mRNA vaccine in patients with a prior COVID-19 infection could be the culprit of her neuro-inflammation. The abbreviated development timeline of COVID-19 vaccines provided little opportunity to identify AEFV [4].
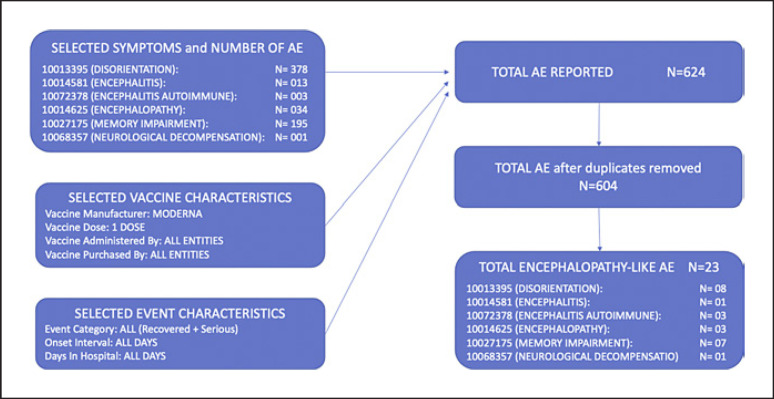
The Vaccine Adverse Events Reporting System (VAERS), part of the US post-marketing vaccine safety surveillance program that monitors vaccine safety and detects rare adverse events (AE) [5], received our patient's report on 3/8/21 (VAERS ID 1082364, E-Report Number 3412289). VAERS raw data of reported AE are publicly available. The VAERS database was searched by the following: vaccine characteristic (COVID-19 Moderna), vaccine manufacturer (Moderna), vaccine dose (first dose), location (all), age (all), sex (all), event category (all events), vaccine administered by (all entities), date report received (from July 1990 to October 2021), symptoms (disorientation, encephalitis, encephalitis autoimmune, encephalopathy, memory impairment, neurological decompensation). The inclusion criteria used were as follows: first dose of Moderna COVID-19 vaccine, encephalopathic symptoms, symptoms lasting more than 24 h, complete description of symptoms, duration of symptoms, outcome. The exclusion criteria used were as follows: severe pain, allergic reactions, flu-like symptoms, gastrointestinal symptoms, hypertensive emergency/urgency, electrolytes abnormalities or metabolic derangement, confirmed acute neurological conditions (ischemic stroke, hemorrhagic stroke, meningitis). Cases were screened by author MR for inclusion. As of October 7th, 2021, from a total of 604 cases retrieved, 23 possible “encephalopathy-like” AEFV after one dose of Moderna COVID-19 vaccine have been identified (Fig. 1). VAERS' data have inconsistent quality and completeness (based on spontaneous reporting) and numerous cases presented as encephalopathy were lacking complete data and did not satisfy our inclusion criteria. A report to VAERS only confirms that the reported event occurred sometime after vaccine was given and causality between vaccine and AEFV cannot be assessed [6].
Fig. 1.
VAERS data. VAERS data concerning “encephalopathy-like” AE after a first injection of Moderna COVID-19 vaccine: from a total of 604 cases retrieved, 23 possible “encephalopathy-like” AE after one dose of Moderna COVID-19 vaccine have been identified. Search strategy, inclusion, and exclusion criteria used for VAERS database search are detailed in the Discussion/Conclusion session.
Possible encephalopathy from the adenoviral vector-based antigen dsDNA Oxford-Astrazeneca Covishield COVID-19 vaccine was reported by mass media [7] as early as December 2020. With the beginning of the global COVID-19 vaccination campaign, a few cases of unexplained encephalopathy following COVID-19 vaccine injections have been reported in literature [8, 9, 10, 11]. The onset of acute encephalopathic symptoms was in all cases within a few days of a COVID-19 vaccine administration, as in our patient, and an extensive metabolic, toxic, infectious, autoimmune work-up was negative. Diffuse electroencephalography slowing was detected in ours and 2 other cases [8, 11]; nonconvulsive status epilepticus was also reported [10]. In prior studies, CSF analysis was unremarkable or showing pleocytosis; one study reported a significant increase of the blood-brain barrier permeability and of cytokines levels in the CSF (interleukin 6 and 8) associated with increased C-reactive protein and interleukin 6 in serum [9]. All patients had an almost complete recovery within a few days of symptom onset. A role for high-dose steroids has been suggested in positive outcome; however, since all patients recovered very rapidly with only 1–2 doses of treatment, it is unclear if the clinical improvement was secondary to steroid use or spontaneous resolution of neuro-inflammation. Here, we report a patient who developed a transient post-COVID-19 vaccine inflammatory encephalopathy in the setting of remote COVID-19 infection (1 year prior), positive SARS-CoV-2 IgG, and elevated inflammatory markers and had a complete and spontaneous resolution of the neurological symptoms on day 5 post-vaccine (day 3 of hospitalization), achieved with no medical treatment.
A rationale for serology-based (IgG-positivity) vaccine dosing and timing has been proposed to maximize population coverage, given the worldwide vaccine shortages [12]. Physicians should be alert to possible postvaccination reactogenicity in individuals with SARS-CoV-2 IgG-positivity, including risk of neuro-inflammation.
Statement of Ethics
Ethics approval was not required for this study in accordance with our SUNY Downstate Medical Center IRB policy on single case reports: this report is granted an exemption from requiring ethics approval. Written consent was obtained from the patient for publication of the details of their medical case as part of the hospital admission process.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contributions
All authors contributed to the study conception and design. Michela Rosso collected the data and wrote the first draft. Yaacov Anziska and Steven R. Levine contributed to review, critique, and substantive revisions.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1.Saadat S, Rikhtegaran Tehrani Z, Logue J, Newman M, Frieman MB, Harris AD, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021 Apr;325((14)):1467–9. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prendecki M, Clarke C, Brown J, Cox A, Gleeson S, Guckian M, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021 Mar;397((10280)):1178–81. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cobo G, Lindholm B, Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant. 2018 Oct;33:iii35–40. doi: 10.1093/ndt/gfy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Tassel K, Shachar C, Hoffman S. Covid-19 vaccine injuries: preventing inequities in compensation. N Engl J Med. 2021 Mar;384((10)):e34. doi: 10.1056/NEJMp2034438. [DOI] [PubMed] [Google Scholar]
- 5.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the vaccine adverse event reporting system (VAERS) Vaccine. 2015 Aug;33((36)):4398–405. doi: 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, et al. First month of COVID-19 vaccine safety monitoring: USA, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021 Feb;70((8)):283–8. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Souza LL. Volunteer's doc blames SII vaccine for neuro disorder [Internet] Mint; 2020. [cited 2021 Nov 19]. Available from: https://www.livemint.com/science/health/volunteer-s-doc-blames-sii-vaccine-for-neuro-disorder-11606880532345.html. [Google Scholar]
- 8.Al-Mashdali AF, Ata YM, Sadik N. Post-COVID-19 vaccine acute hyperactive encephalopathy with dramatic response to methylprednisolone: a case report. Ann Med Surg. 2021 Sep;69:102803. doi: 10.1016/j.amsu.2021.102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldelli L, Amore G, Montini A, Panzera I, Rossi S, Cortelli P, et al. Hyperacute reversible encephalopathy related to cytokine storm following COVID-19 vaccine. J Neuroimmunol. 2021 Sep;358:577661. doi: 10.1016/j.jneuroim.2021.577661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu BD, Ugolini C, Jha P. Two cases of post-Moderna COVID-19 vaccine encephalopathy associated with nonconvulsive status epilepticus. Cureus. 2021 Jul;13((7)):e16172. doi: 10.7759/cureus.16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuhorn F, Graf T, Klingebiel R, Schäbitz WR, Rogalewski A. Postvaccinal encephalitis after ChAdOx1 nCov-19. Ann Neurol. 2021 Sep;90((3)):506–11. doi: 10.1002/ana.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manisty C, Otter AD, Treibel TA, McKnight Á, Altmann DM, Brooks T, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021 Mar;397((10279)):1057–8. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.