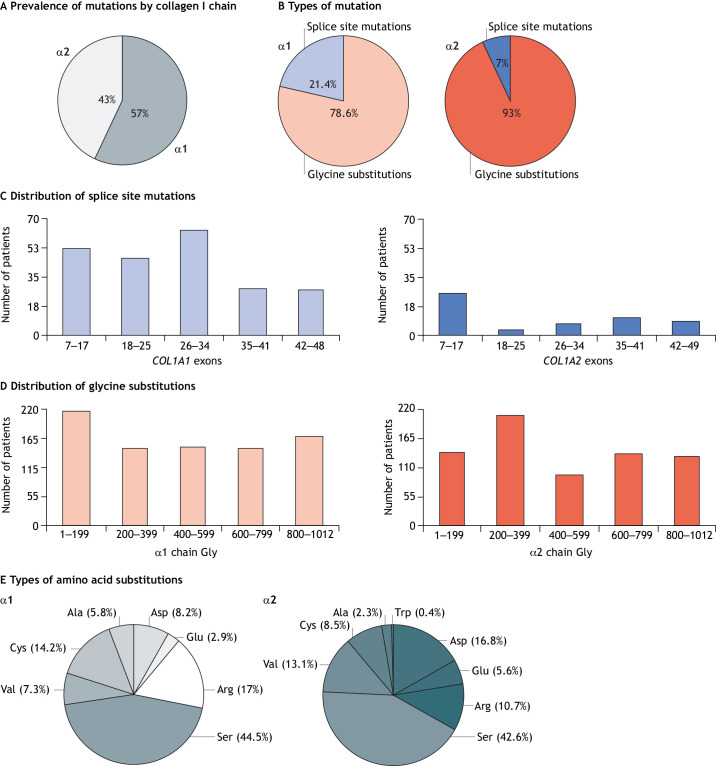
Fig. 2.
Distribution of collagen type I sequence variants in α1(I) and α2(I) chains, type/position of the variants and nature of the substituting amino acid in osteogenesis imperfecta (OI). (A) Overall distribution of sequence variants in collagen I chains. (B) Distribution of glycine substitutions and splice site sequence variations in the α1(I) and α2(I) chains of collagen I. Glycine substitutions are shown in red, splice site mutations are shown in blue. (C) Distribution of splice site sequence variants along the COL1A1 and COL1A2 genes. (D) Distribution of glycine (Gly) substitution sequence variants along the α1(I) and α2(I) chains. (E) Distributions of bulkier amino acids substituting glycine in the α1(I) and α2(I) chains, resulting in structure alterations. These panels summarize data from our new analysis of the public COL1A1 and COL1A2 sequence variant databases. We focus on OI causative variants that affect the triple-helix region of collagen type I. COL1A1: https://databases.lovd.nl/shared/genes/COL1A1; COL1A2: https://databases.lovd.nl/shared/genes/COL1A2.