SUMMARY
Shiga toxin-producing Escherichia coli (STEC) is an important cause of gastroenteritis (GE) and haemolytic uraemic syndrome (HUS). Incidence of STEC illness is largely underestimated in notification data, particularly of serogroups other than O157 (‘non-O157’). Using HUS national notification data (2008–2012, excluding 2011), we modelled true annual incidence of STEC illness in Germany separately for O157 and non-O157 STEC, taking into account the groups’ different probabilities of causing bloody diarrhoea and HUS, and the resulting difference in their under-ascertainment. Uncertainty of input parameters was evaluated by stochastic Monte Carlo simulations. Median annual incidence (per 100 000 population) of STEC-associated HUS and STEC-GE was estimated at 0·11 [95% credible interval (CrI) 0·08-0·20], and 35 (95% CrI 12-145), respectively. German notification data underestimated STEC-associated HUS and STEC-GE incidences by factors of 1·8 and 32·3, respectively. Non-O157 STEC accounted for 81% of all STEC-GE, 51% of all bloody STEC-GE and 32% of all STEC-associated HUS cases. Non-O157 serogroups dominate incidence of STEC-GE and contribute significantly to STEC-associated HUS in Germany. This might apply to many other countries considering European surveillance data on HUS. Non-O157 STEC should be considered in parallel with STEC O157 when searching aetiology in patients with GE or HUS, and accounted for in modern surveillance systems.
Key words: Burden of illness, enterohaemorrhagic Escherichia coli, Escherichia coli O157, incidence, Monte Carlo method
INTRODUCTION
Shiga toxin-producing Escherichia coli (STEC) is an important cause of gastroenteritis (GE) and life-threatening haemolytic uraemic syndrome (HUS) in many countries. STEC has a zoonotic reservoir (mainly ruminants) and is transmitted by inadvertent ingestion of small amounts of faecal matter. The serotype is an indicator of the genomic strain content and incidence of human illness and disease severity varies by serotype [1, 2]. Evidence from observational studies suggests that STEC of serogroup O157 with serotypes H7 or H- (O157 STEC) are, on average, substantially more virulent than other (‘non-O157’) STEC implicated with human illness [2–4]. O157 STEC is the leading cause of paediatric HUS [5] and the most frequently isolated aetiological agent in STEC outbreaks worldwide [6]. These organisms can be easily identified by culture on selective and differential agar [7], with the exception of rarely identified sorbitol-fermenting (sf) clones [8, 9].
Non-O157 STEC represents a genomically heterogeneous group of organisms, comprising STEC with little or no virulence to humans but also with high virulence, e.g. STEC O104:H4 that caused the largest outbreak of HUS thus far [10]. Currently, diagnosis of non-O157 STEC is more complex and requires screening for Shiga toxins or their encoding genes. Culture isolation and subsequent serotyping is often conducted only at public health laboratories. Diagnosis of non-O157 STEC is disproportionately underutilized, even in countries where their diagnosis is recommended. Consequently, surveillance for non-O157 STEC in many countries is less inclusive than for O157 STEC and their contribution to incidence of STEC illness has been insufficiently determined.
Notification data, including statutory, capture only a fraction of illnesses that are occurring in the population. In Germany, median annual incidence (per 100 000 population) of notified cases is 0·06 for STEC-associated HUS (and 1·07 for STEC-GE) for 2008–2012, excluding 2011 (https://survstat.rki.de, data version 1 July 2014).
Studies addressing underestimation in notification data and the quantitative relation of non-O157 STEC to O157 STEC are helpful in informing diagnostic and surveillance strategies – as were previous studies for other gastroenteric pathogens [11].
The few available studies suggest a true annual incidence of STEC-associated infections between 47 and 100/100 000 population for Europe [12] and Northern America [13, 14] and 0·15 STEC-associated HUS [12]. Estimated proportions of non-O157 in STEC-GE were 62% and 64% in Canada [14] and the United States [13], respectively. All available studies extrapolated data from different countries or data on other pathogens than STEC for their estimation models [12–14], thus introducing a further source adding to the inherent uncertainty of stochastic modelling. Furthermore, estimates of overall STEC-GE and the proportion of O157 STEC are based, at best, on STEC-GE surveillance data [13] with all of its diagnostic vagaries mentioned above, or on assumptions [12, 14] but not on HUS statutory surveillance data.
Our objectives were to estimate annual frequency and incidence of STEC-associated HUS and STEC-GE in Germany based on German national notification data for enteropathic HUS – overall and separately for O157 STEC and non-O157 STEC – to inform diagnostic, and surveillance strategies.
METHODS
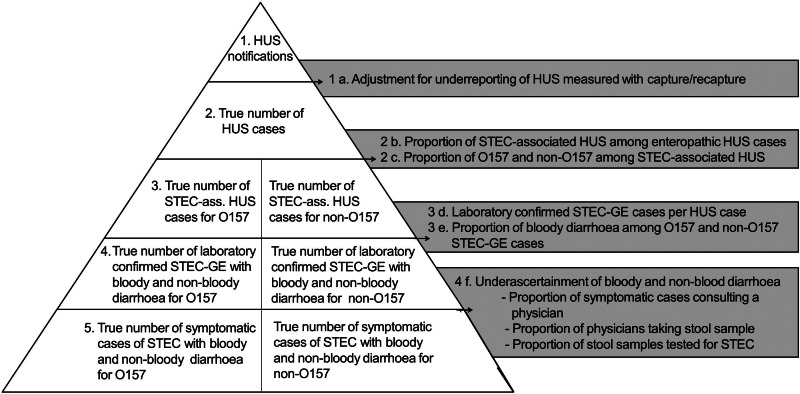
Using HUS national notification data as a starting point, we modelled true annual incidence of STEC illness in Germany separately for O157 and non-O157 STEC, taking into account group-specific underestimation caused by underreporting of notification data and under-ascertainment (see Fig. 1).
Fig. 1.
Modelling true annual incidence of O157 and non-O157 STEC illness in Germany based on notification data of haemolytic uraemic syndrome (HUS).
Diagnosis and surveillance of STEC-GE and ‘enteropathic’ HUS in Germany
In Germany, diagnosis of STEC in GE and HUS patients is based on detection of Shiga toxins or their encoding genes in stool enrichment culture or isolates. Subsequent culture isolation and serotyping is recommended but not mandatory and is rarely performed in clinical laboratories. In HUS patients, evidence for an STEC infection can also be established by detecting anti-lipopolysacharide IgM antibodies against E. coli serogroups in blood by specialized laboratories (which in the study period included only antibodies against serogroup O157).
According to the German Protection against Infection Act, both laboratory detection of STEC infection in stool and clinically diagnosed ‘enteropathic’ (i.e. GE-associated) HUS are notifiable (see Supplementary material for national surveillance case definitions).
Electronic case reports are sent from the local health department via State Health Departments to the federal-level public health institute, the Robert Koch Institute (RKI), where reports are hosted in a national database. In addition, RKI conducts active surveillance for paediatric HUS since 2008 in collaboration with the German Society for Paediatric Nephrology. This surveillance entails monthly inquiries to all paediatric nephrology centres (PNCs) in Germany about incident HUS cases in children (aged <18 years) during the past month.
Risk model for STEC illness in Germany
We used German notification data on enteropathic HUS, reported to RKI for the years 2008–2012 (excluding 2011 because of a large outbreak of STEC O104:H4 [10]) as the basis to estimate the true annual incidence of STEC-GE in Germany.
We computed estimates separately for O157 and non-O157 STEC groups, taking into account the groups’ different average capability of causing acute bloody diarrhoea [15] and HUS, and the resulting difference in under-ascertainment caused by symptomatic cases not attending health facilities (for differences in clinical severity) or by not being correctly diagnosed as a case (for differences in diagnostics as outlined above). Furthermore, underreporting of cases from health facilities to public health authorities adds to underestimation of STEC-GE incidence.
Our estimations were conducted in the following sequence (see also Fig. 1):
Adjustment for underreporting of HUS. To estimate the true median annual numbers of enteropathic HUS, adjustment for underreporting was conducted separately for cases treated in PNCs and non-PNCs. For PNCs, we used a two-source capture–recapture approach (statutorily passive HUS surveillance and active paediatric HUS surveillance) to estimate the magnitude of underreporting of notification data. We assumed underreporting by non-PNCs to be up to ten times more common than in PNCs as HUS cases are infrequently treated in these institutions. Consequently, knowledge of infectious disease notification requirements, otherwise seldom needed in nephrology units, is likely to be less prevalent in medical personnel in non-PNCs.
Estimating the proportion of STEC-associated HUS. Evidence of STEC infection cannot be established in every case of ‘enteropathic’ HUS. Using literature (described in detail in the Supplementary material) on microbiological evidence of STEC in HUS patients in Germany, we estimated the proportion of enteropathic HUS caused by STEC infection [16]. This proportion was subsequently multiplied by the estimated number of all HUS cases per year to obtain the number of estimated STEC-associated HUS cases.
Estimating the proportion of O157 and non-O157 in STEC-associated HUS. The proportion of O157 in STEC-associated HUS in Germany was derived from the literature [16, 17] and combined as outlined in Table 1. This proportion was multiplied by the annual number of STEC-associated HUS cases to estimate the O157-associated HUS cases (the remaining STEC-HUS cases were thus non-O157 associated). All further calculations were conducted separately for O157- and non-O157-associated HUS cases.
Estimating the number of laboratory-confirmed STEC-GE cases per HUS case. Using literature information on the proportion of HUS cases in laboratory-confirmed STEC-GE cases [18], we multiplied the estimated annual number of STEC-associated HUS cases by the factor for STEC-GE cases per STEC-associated HUS case separately for O157 and non-O157 (beta distribution).
Estimating the proportion of bloody diarrhoea in O157 and non-O157 STEC-GE cases. In addition, we used the literature for estimates on the proportion of bloody diarrhoea in O157 and non-O157 STEC-GE cases [18]. Annual frequencies for STEC-GE with bloody and non-bloody diarrhoea were used to account for under-ascertainment according to severity in a next step (separately for O157 and non-O157).
Estimating the under-ascertainment of bloody and non-bloody diarrhoea. Under-ascertainment was accounted for in a procedure incorporating three steps: using literature information, we first estimated the proportion of symptomatic patients consulting a physician, thereafter the proportion of patients that provided stool specimens for microbiological testing [19, 20] and finally the proportion of stool samples tested for STEC [21] based on German laboratory recommendations on test strategies for faecal samples [21].
The estimated annual number of true STEC-GE cases and STEC-associated HUS cases in Germany, differentiated for O157 and non-O157, were converted to annual cumulative incidence/100 000 population, using the mean population size of Germany for 2008–2012 (excluding 2011), obtained from Germany's Federal Statistical Office.
Table 1.
Input parameters for the risk model to estimate true incidence of O157 and non-O157 STEC illness in Germany based on notification data of haemolytic uraemic syndrome (HUS)
| Steps in estimation | Parameters | S* | N† | Distribution‡ | Median | 95% CrI | Source |
|---|---|---|---|---|---|---|---|
| 1. HUS notifications | Incidence of notified cases | 260 | 327 × 108§ | Gamma(260, 3 × 10−9) | 8 × 10−7 | 7 × 10−7–9 × 10−7 | German notification data |
| (a) Adjustment for underreporting separately for cases treated in PNCs and non-PNCs | Proportion of HUS-notifications treated by PNCs | 153 | 254 | Beta(154, 102) | 0·60 | 0·54–0·66 | National active and passive surveillance, unpublished |
| Completeness of HUS-notification from PNCs | 153 | 183 | Beta(154, 31) | 0·83 | 0·78–0·88 | National active and passive surveillance, unpublished | |
| Multiplication factor to extrapolate completeness of notification from PNCs to non-PNCs | − | − | Pert(0·1, 0·5, 1) | 0·51 | 0·21–0·84 | Assumption | |
| (b) Proportion of STEC-associated HUS in enteropathic HUS cases | Proportion of STEC-associated-HUS | 327 | 394 | Beta(328, 68) | 0·83 | 0·79–0·86 | Gerber et al. 2002 [16] |
| (c) Proportion of O157 and non-O157 in STEC-associated HUS | Proportion of O157 in STEC-associated HUS | 138 | 207 | Beta(494, 239) | 0·67 | 0·64–0·71 | Gerber et al. 2002 [16] |
| 355 | 524 | Mellmann et al. 2008 [17] | |||||
| (d) Number of laboratory-confirmed STEC-GE cases per HUS case | Proportion HUS in laboratory-confirmed O157 STEC | 3 | 27 | Beta(4, 25) | 0·13 | 0·04–0·28 | Werber et al. 2007 [18] |
| Proportion HUS in laboratory-confirmed non-O157 STEC | 2 | 149 | Beta(3, 148) | 0·02 | 0·00–0·05 | Werber et al. 2007 [18] | |
| (e) Proportion of bloody diarrhoea in O157 and non-O157 in STEC-GE cases | Proportion of cases experiencing bloody O157-associated diarrhoea | 10 | 27 | Beta(11, 18) | 0·38 | 0·22–0·56 | Werber et al. 2007 [18] |
| Proportion of cases experiencing bloody non-O157-associated diarrhoea | 16 | 149 | Beta(17, 134) | 0·11 | 0·07–0·17 | Werber et al. 2007 [18] | |
| (f) Underascertainment of bloody and non-bloody diarrhoea | Proportion of patients visiting physicians with bloody diarrhoea | 21 | 41 | Beta(22, 21) | 0·51 | 0·36–0·66 | Haagsma et al. 2013 [19] |
| Proportion of patients visiting physicians with non-bloody diarrhoea | 458 | 1342 | Beta(555, 1093) | 0·34 | 0·31–0·36 | Haagsma et al. 2013 [19] | |
| 96 | 304 | Hauri et al. 2011 [20] | |||||
| Proportion of physicians taking laboratory samples from patients with bloody diarrhoea | 10 | 20 | Beta(11, 11) | 0·50 | 0·30–0·70 | Haagsma et al. 2013 [19] | |
| Proportion of physicians taking laboratory samples from patients with non-bloody diarrhoea | 155 | 456 | Beta(170, 383) | 0·31 | 0·27–0·35 | Haagsma et al. 2013 [19] | |
| 14 | 95 | Hauri et al. 2011 [20] | |||||
| Proportion of stool samples tested for STEC from patients with bloody diarrhoea | − | − | None | 1·00 | 1·00–1·00 | Kist et al. 2013 [21] | |
| Proportion of stool samples tested for STEC from patients with non-bloody diarrhoea | − | − | Pert(0·1, 0·8, 1) | 0·74 | 0·37–0·96 | Kist et al. 2013 [21], assumption |
CrI, Credible interval; PNC, paediatric nephrology centre.
Nominator.
Denominator.
The unit of measurement is person-years-at-risk for this parameter.
For Gamma(r, λ), r = s and λ = 1/N; for Beta(a, b), a = Sum(s) + 1 and b = Sum(N) – Sum(s) + 1.
Evaluation of uncertainty
We used Monte Carlo simulation in @RISK v. 6.1.1 (Palisade Corp., USA) with Latin Hypercube sampling and 10 000 iterations to evaluate uncertainty in the outputs. All input data was considered to be subject to uncertainty and parameters were therefore described by probability distributions. Generally, proportions were described by beta distributions and the HUS rate was described by a gamma distribution [22]. Pert distributions were used for multiplication factors where sufficient data to inform beta distributions was unavailable. Distribution parameterization was done as displayed in Table 1. The results are reported as the median and the 95% credible interval.
A sensitivity analysis was conducted to evaluate the contribution of the input parameters to the overall uncertainty in outcome estimates to identify which input parameter shows the biggest influence on the output.
In addition we examined two scenarios using alternative values of particularly uncertain input parameters to investigate their effect on the outcome estimates, keeping all other variables of the model constant. (For details see Supplementary material.) In a conservative scenario (scenario 1) we assumed that degree of underreporting of HUS did not differ between PNCs and non-PNCs and that all stool samples submitted for microbiological testing were investigated for STEC regardless of whether blood was visible. In scenario 2 we re-parameterized the model using input parameters for under-ascertainment based on findings of a survey in the Federal state of Hesse in children aged <16 years [20], to account for under-ascertainment in our estimates of the higher incidence of STEC illness in children.
Literature survey
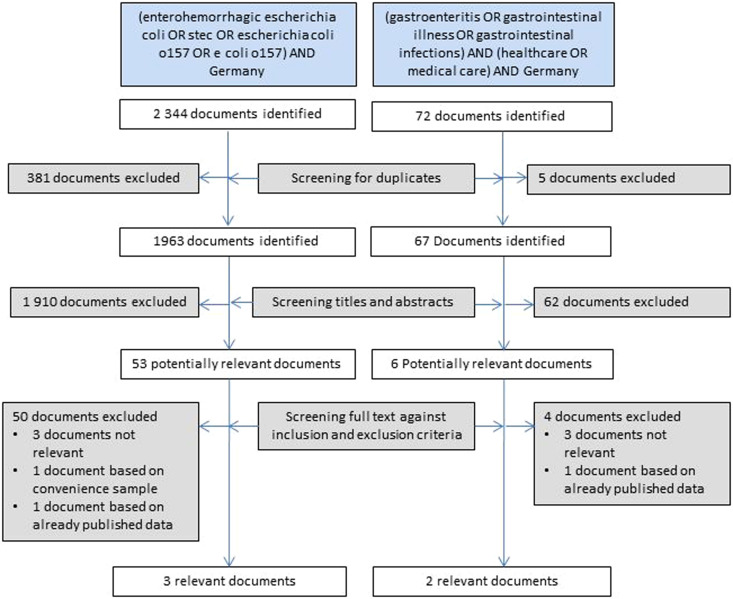
We searched Medline and Scopus literature for information about STEC in Germany published since inception of the Medline and Scopus bibliographic databases to 31 December 2014 with the objective of identifying, for patients in Germany, the proportion of STEC-associated HUS in enteropathic HUS cases (as input parameter for estimation step b), the proportion of O157 STEC in STEC-associated HUS (step c) and the proportion of HUS and bloody diarrhoea in laboratory-confirmed STEC-GE separately for O157 and non-O157 serogroups (step d). Our second objective was to identify under-ascertainment of bloody and non-bloody diarrhoea (step f), including the proportion of physician consultations in cases of bloody and non-bloody diarrhoea and the proportion of physicians taking stool samples in cases of bloody and non-bloody diarrhoea.
We used the search terms (enterohaemorrhagic Escherichia coli OR STEC OR Escherichia coli O157 OR E. coli O157) AND (Germany) to identify input parameters for steps b–e. We used search terms (gastroenteritis OR gastrointestinal illness OR gastrointestinal infections) AND Germany AND (healthcare OR medical care) in titles and abstracts for step f.
We required articles for all steps to provide data in sufficient detail for O157 and non-O157 regarding proportion of HUS and bloody diarrhoea and to refer to data that pertained to Germany recognizing that serogroup distribution among GE and HUS cases as well as health-seeking behaviour may vary between countries. In addition, we required information for steps d–f to be derived from population-based surveys or sentinel surveillance projects to increase accuracy of these estimates. Search results for Medline and Scopus were combined and de-duplicated. Two investigators screened documents independently, in the event of discrepancies consensus by discussion was sought. Documents were first screened by reviewing titles and abstracts were available. Identified documents were screened against inclusion and exclusion criteria outlined above. From the identified documents absolute numbers were extracted and used as input variables in the estimation model as outlined in Table 1.
RESULTS
We identified five relevant publications, three for steps b–e and two for step f [16–20] that together provided information for all required input parameters (see Fig. 2 and Supplementary material). These publications, German notification data and German laboratory guidelines formed the backbone of the simulation model and are outlined in Table 1.
Fig. 2.
Results of the systematic review to identify input parameters for the estimation of the true incidence of O157 and non-O157 STEC illness in Germany based on notification data of haemolytic uraemic syndrome (HUS).
We estimated a median annual number of 90 cases of STEC-associated HUS in Germany during the study period, corresponding to an incidence (per 100 000 population) of 0·11 [95% credible interval (CrI) 0·08–0·20]; a median of 60 cases due to STEC O157 (incidence 0·07, 95% CrI 0·05–0·13) and a median of 29 cases due to non-O157 STEC (incidence 0·04, 95% CrI 0·03–0·07) (see Table 2). From these, we estimated that a median of 28 347 STEC-GE cases occurred per year in the German population, indicating an incidence of 35 (95% CrI 12–145); a median of 4969 cases due to O157 STEC (incidence 6.1, 95% CrI 2·2–24) and a median of 22 019 cases due to non-O157 STEC (incidence 27, 95% CrI 8·0–133).
Table 2.
Results of modelling median annual case numbers and median annual incidence (with 95% credible intervals) of O157 and non-O157 STEC illness in Germany based on notification data of haemolytic uraemic syndrome (HUS)
| No. | Total | O157 total | O157 bloody GE | O157 non-bloody GE | Non-O157 total | Non-O157 bloody GE | Non-O157 non-bloody GE | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | HUS notifications | Median | 65 (58–74) | ||||||
| Incidence* | 0·08 (0·07–0·09) | ||||||||
| 2 | Estimated true number of HUS cases | Median | 108 (80–197) | ||||||
| Incidence* | 0·13 (0·10–0·24) | ||||||||
| 3 | Estimated true number of STEC-associated HUS cases | Median | 90 (66–164) | 60 (44–110) | 29 (21–54) | ||||
| Incidence* | 0·11 (0·08–0·20) | 0·07 (0·05–0·13) | 0·04 (0·03–0·07) | ||||||
| 4 | Estimated true number of laboratory- confirmed symptomatic STEC cases | Median | 2312 (954–8864) | 486 (199–1705) | 181 (63–678) | 299 (116–1079) | 1706 (577–7999) | 189 (58–908) | 1514 (513–7096) |
| Incidence* | 2·83 (1·20–10·80) | 0·59 (0·24–2·08) | 0·22 (0·08–0·83) | 0·37 (0·14–1·32) | 2·09 (0·70–9·80) | 0·23 (0·07–1·11) | 1·85 (0·60–8·7) | ||
| 5 | Estimated true number of symptomatic STEC cases | Median | 28 347 (10 217–119 041) | 4969 (1835–19 406) | 730 (229–3037) | 4171 (1449–16 846) | 22 019 (6764–109 046) | 769 (211–3,925) | 21 192 (6481–105 641) |
| Incidence* | 34·63 (12·00–145·00) | 6·07 (2·2–23·7) | 0·89 (0·28–3·71) | 5·10 (1·80–20·60) | 26·90 (8·00–133·00) | 0·94 (0·26–4·80) | 25.89 (8·00–129·00) |
Per 100 000 population.
Our estimates correspond to a median annual underestimation of STEC-associated HUS and STEC-GE in the German notification data by a factor of 1·8 (95% CrI 1·3–3·3) and 32 (95% CrI 11–135), respectively.
Non-O157 STEC accounted for 81% (95% CrI 49–96) of all STEC-GE and 51% (95% CrI 16–86) of all bloody STEC-associated diarrhoea.
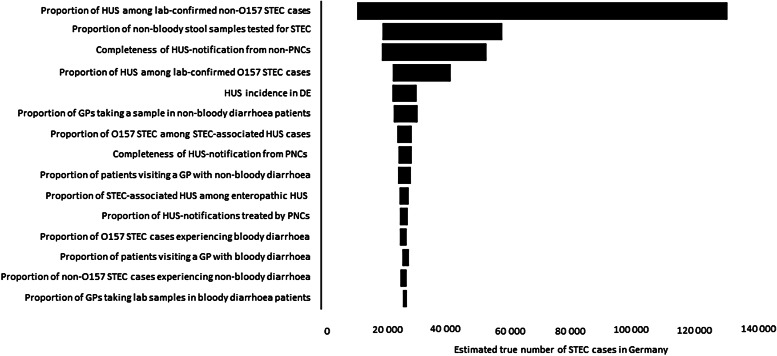
Sensitivity analysis indicated that the proportion of HUS cases in laboratory-confirmed non-O157 STEC exerted the biggest influence on the outcome of all input parameters, followed by the proportion of stool samples tested for STEC and the completeness of HUS notifications from non-PNCs (see Fig. 3).
Fig. 3.
Sensitivity analysis of influence of input parameters on frequency of STEC-GE in Germany based on notification data of haemolytic uraemic syndrome (HUS).
In scenario analysis, the median annual incidence (per 100 000 population) of STEC-GE ranged from 17 (95% CrI 7·6–61) in scenario 1 to 72 (95% CrI 22–339) in scenario 2 and of STEC-associated HUS from 0·08 (95% CrI 0·07–0·09) in scenario 1 to 0·11 (95% CrI 0·08–0·20) in scenario 2 (unchanged to the point estimate).
The proportion of non-O157 STEC in STEC-GE, bloody diarrhoea and STEC-associated HUS did not vary in the different scenarios (see Supplementary material for detailed results).
DISCUSSION
We estimated the true frequency and incidence of STEC illness in the German population, separately for O157 and non-O157 STEC, based on statutory notification data on HUS. The study yielded the following main findings: The median annual incidence (per 100 000 population) was estimated at 35 (95% CrI 12–145·00) for STEC-GE and 0·11 for STEC-associated HUS (95% CrI 0·08–0·20). German notification data underestimated STEC-associated HUS and STEC-GE incidences by factors of 1·8 and 32·3, respectively. Non-O157 STEC accounted for ~80% of all STEC-GE, half of all bloody STEC-associated diarrhoea and one-third of all STEC-associated HUS cases, hence contributing to STEC illness to an even larger extent than previously estimated [13, 14].
Our incidence point estimates for STEC-GE and HUS are slightly lower than those published for Europe (47 and 0·15, respectively) [12], the United States (59 for STEC-GE) [13] and Canada (100 for STEC-GE) [14], but in keeping considering the degree of uncertainty accompanying our estimate. The incidence for O157 STEC-GE is in particular lower than estimated for other European countries such as The Netherlands [19, 23], Denmark or the UK [19], and for the United States and Canada [12, 13]. In Germany, neither laboratory-based (passive) surveillance of STEC-GE nor (active) HUS surveillance ever identified an outbreak with ‘classical’ non-sf O157 STEC comprising ⩾5 persons, but did so for outbreaks with other serotypes [24, 25]. We are unaware of specific control plans for O157 STEC in animal reservoirs or the food-production chain that would explain this observation. Thus, our estimation of a comparatively low O157 STEC incidence adds additional weight to the view that O157 STEC poses a limited public health problem in Germany.
Of note, according to surveillance data (2008–2012, excluding 2011) reported to the European Centre for Disease Control and Prevention (ECDC) from other countries in the European Union, a slightly higher percentage (40%, 391/659) of all STEC identified in reported HUS patients belonged to non-O157 serogroups (data provided by ECDC extracted from The European Surveillance System; TESSy). This may indicate that non-O157 STEC contribute to STEC-GE incidence in other European countries even more than in Germany (where non-O157 STEC account for 80% of STEC-GE). Yet, only 33% of STEC-GE captured in surveillance systems in Europe were attributed to infection by non-O157 strains during the study period [26, 27], underscoring the large degree of under-ascertainment of these STEC strains in GE patients in Europe. In recent years, the proportion of non-O157 STEC increased, probably indicating a more frequent use of serogroup-independent testing in Europe [26, 27].
In Germany, the contribution of the different non-O157 serogroups to STEC illness has remained fairly constant over the last 10 years (except in 2011) according to German surveillance data with serogroups O26, and O103 being the most frequently isolated non-O157 STEC in children and O91 in adults [18, 28]. The numerous different non-O157 STEC vary markedly in their virulence. On average though, they less frequently causes life-threatening HUS (in children) or disease outbreaks, and, importantly, their diagnosis currently is more complex, time-consuming and expensive. Thus, the question about the cost-effectiveness of screening for non-O157 has been raised [29, 30]. Apart from their markedly more frequent occurrence as an aetiological agent in human GE than STEC O157 and their substantial contribution to the burden of bloody diarrhoea and HUS, new STEC strains are likely to evolve, some of which will cause outbreaks (e.g. STEC O104:H4) [10]. For the latter reason alone we believe that modern STEC diagnosis and, consequently, surveillance systems should encompass timely detection of non-O157 STEC (including information on the serotype or other epidemiologically meaningful subtyping information), even in countries where STEC O157 appears to dominate.
Validity of risk model
Our ‘top-down’ approach of estimating STEC incidence based on HUS notification data is new and we believe is advantageous for at least two reasons. First, statutory HUS surveillance is more sensitive than STEC-GE surveillance and in conjunction with active paediatric HUS surveillance in Germany allowed for an accurate estimate of its underreporting. Furthermore, STEC aetiology in (paediatric) HUS patients has been extensively studied in Germany [16, 17]. Taken together, HUS incidence and the individual contribution of O157 and non-O157 STEC could be estimated with little uncertainty.
Second, our estimations were purposively based solely on information on STEC in Germany, preventing the need of extrapolating from data gathered in other countries as another source of uncertainty.
By far the greatest source of uncertainty was the proportion of HUS in patients infected by a non-O157 STEC because it was based on small numbers. However, our estimate is in agreement with data from other countries [31]. Likewise, other findings are corroborated by data sources not used in our estimation. For example, the estimated proportion of non-O157 STEC-associated HUS (33%) is consistent with that observed in national HUS notification data during the study period (34%). Furthermore, the proportion of non-O157 serogroups in STEC-GE and STEC-associated bloody diarrhoea in Germany is consistent with both national notification data on STEC-GE and with a nationwide laboratory sentinel conducted at the beginning of the century in Germany [18].
Limitations
As with previously published risk models, ours did not account for the effect of age because age-specific data were unavailable for many estimation steps. Yet, the serogroup-specific incidence for STEC-GE and HUS incidence vary with age. Most available studies focused exclusively or primarily on children (who should have the highest true incidence of STEC-GE and HUS in Germany), which is why uncertainty of estimates is likely highest for adults.
In addition, non-O157 STEC consist of different pathogens with a variety of virulence genes, and estimates for non-O157 relate to the fairly stable serogroup distribution (and assumed average genomic content within serogroups) in Germany, which can be different in other countries. Models based on virulence-genes might be preferable but the necessary input data (e.g. stx-type, presence of the eae gene) are currently not available in sufficient detail.
Furthermore, some input data of our risk model lack an evidence base as no study was available to support our assumptions, such as underreporting from non-PNCs and the adherence to laboratory guidelines for testing stool samples of GE cases. These two parameters were in the top-3 influential parameters in the sensitivity analysis, warranting further data collection to decrease this uncertainty. Furthermore, not all literature sources used for our risk model distinguished between (rare) sf-O157 STEC and (‘classic’) non-sf-O157 STEC. Because sf-O157 STEC infection progresses with a higher probability from diarrhoea to HUS [32], we slightly overestimated STEC-GE incidence of serogroup O157.
Completeness of HUS notification is probably overestimated in our study because concurrently conducted active paediatric surveillance included reminders of notification obligations when continuously monitoring HUS cases ascertained in the active system.
CONCLUSIONS
Statutory notification data largely underestimate STEC-GE in Germany, where STEC diagnosis is based on serogroup-independent testing for Shiga toxins or their encoding genes.
The contribution of non-O157 serogroups to STEC-GE incidence appears to be higher than previously estimated [13, 14], not only including a large number of mild illnesses but also half of all STEC-associated bloody diarrhoea cases. Considering European surveillance data on HUS, this finding is probably true for many other countries in Europe. Surveillance of HUS complements that of STEC-GE, not only by allowing for the detection of outbreaks that otherwise go unrecognized [33] and reliably monitoring trends of STEC infection [34], but also by aiding in estimating STEC incidence, thereby helping to validate notification data.
Non-O157 STEC should be considered in parallel with STEC O157 when searching aetiology in patients with GE or HUS, and accounted for in modern surveillance systems for STEC illness.
ACKNOWLEDGEMENTS
The authors thank Anja Hauri for providing data on a survey in the German Federal state of Hesse. The authors are grateful for data provided by ECDC extracted from The European Surveillance System (TESSy).
APPENDIX. Collaborators of the HUS active surveillance network Germany
Oliver Amon (University Hospital Tuebingen, Tuebingen), Rainer Büscher (University Hospital Essen, Essen), Tobias Hampel (Children's Hospital Memmingen, Memmingen), Henry Fehrenbach (Children's Hospital Memmingen, Memmingen), Sandra Habbig (University Hospital of Cologne, Cologne), Martin Pohl (University Hospital Freiburg, Freiburg), Karsten Häffner (University Hospital Freiburg, Freiburg), Bernd Hoppe (University Hospital Bonn, Bonn), Günter Klaus (University Children's Hospital, Marburg), Martin Konrad (University Children's Hospital, Münster), Kay Latta (Clementine Children's Hospital, Frankfurt), Heinz Leichter (Olgahospital, Stuttgart), Sebastian Loos (University Medical Center Hamburg-Eppendorf, Hamburg), Carmen Montoya (Children's Hospital Schwabing, München), Dominik Müller (Berlin Medical University Centre ‘Charité’), Matthias Galiano (Medical University, Erlangen), Evelin Muschiol (Medical University, Erlangen), Lars Pape (Hannover Medical School, Hannover), Hagen Staude (University Children's Hospital Rostock, Rostock), Elke Wühl (Center for Pediatrics and Adolescent Medicine, Heidelberg), Michael Henn (St Georg Hospital, Leipzig), Simone Wygoda (St Georg Hospital, Leipzig), Michael Pohl (Children's Hospital Friedrich Schiller University Jena, Jena).
DECLARATION OF INTEREST
None.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268816001436.
click here to view supplementary material
REFERENCES
- 1.Boerlin P, et al. Associations between virulence factors of shiga toxin-producing Escherichia coli and disease in humans. Journal of Clinical Microbiology 1999; 37: 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preussel K, et al. Shiga toxin-producing Escherichia coli O157 is more likely to lead to hospitalization and death than non-O157 serogroups – except O104. PLoS ONE 2013; 8: e78180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadler JL, et al. Ten-year trends and risk factors for non-o157 shiga toxin-producing Escherichia coli found through shiga toxin testing, Connecticut, 2000–2009. Journal of Clinical Infectious Diseases 2011; 53: 269–276. [DOI] [PubMed] [Google Scholar]
- 4.Hedican EB, et al. Characteristics of O157 versus non-O157 shiga toxin-producing Escherichia coli infections in Minnesota, 2000–2006. Journal of Clinical Infectious Diseases 2009; 49: 358–364. [DOI] [PubMed] [Google Scholar]
- 5.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005; 365: 1073–1086. [DOI] [PubMed] [Google Scholar]
- 6.Donnenberg MS, Whittam TS. Pathogenesis and evolution of virulence in enteropathogenic and enterohemorrhagic Escherichia coli. Journal of Clinical Investigation 2001; 107: 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gould LH, et al. Recommendations for diagnosis of shiga toxin-producing Escherichia coli infections by clinical laboratories. Morbidity and Mortality Weekly Reports 2009; 58: 1–14. [PubMed] [Google Scholar]
- 8.Bielaszewska M, et al. Phenotypic and molecular analysis of tellurite resistance among enterohemorrhagic Escherichia coli O157:H7 and sorbitol-fermenting O157:Nm clinical isolates. Journal of Clinical Microbiology 2005; 43: 452–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werber D, et al. Watch out for the even eviler cousin – sorbitol-fermenting E. coli O157. Lancet 2011; 377: 298–299. [DOI] [PubMed] [Google Scholar]
- 10.Frank C, et al. Epidemic profile of shiga-toxin-producing Escherichia coli O104:H4 outbreak in germany. New England Journal of Medicine 2011; 365: 1771–1780. [DOI] [PubMed] [Google Scholar]
- 11.Havelaar AH, et al. Disease burden of foodborne pathogens in the Netherlands, 2009. International Journal of Food Microbiology 2012; 156: 231–238. [DOI] [PubMed] [Google Scholar]
- 12.Majowicz SE, et al. Global incidence of human shiga toxin-producing Escherichia coli infections and deaths: a systematic review and knowledge synthesis. Foodborne Pathogens and Disease 2014; 11: 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scallan E, et al. Foodborne illness acquired in the united states – major pathogens. Emerging Infectious Diseases 2011; 17: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas MK, et al. Estimates of the burden of foodborne illness in Canada for 30 specified pathogens and unspecified agents, circa 2006. Foodborne Pathogens and Disease 2013; 10: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werber D, et al. Shiga toxin-producing Escherichia coli O157 more frequently cause bloody diarrhea than do non-O157 strains. Journal of Infectious Diseases 2004; 189: 1335–1336; author reply 1336–1337. [DOI] [PubMed] [Google Scholar]
- 16.Gerber A, et al. Clinical course and the role of shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997–2000, in Germany and Austria: a prospective study. Journal of Infectious Diseases 2002; 186: 493–500. [DOI] [PubMed] [Google Scholar]
- 17.Mellmann A, et al. Analysis of collection of hemolytic uremic syndrome-associated enterohemorrhagic Escherichia coli. Emerging Infectious Diseases 2008; 14: 1287–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werber D, et al. Shiga toxin-producing Escherichia coli infection in Germany: Different risk factors for different age groups. American Journal of Epidemiology 2007; 165: 425–434. [DOI] [PubMed] [Google Scholar]
- 19.Haagsma JA, et al. Community incidence of pathogen-specific gastroenteritis: Reconstructing the surveillance pyramid for seven pathogens in seven European Union member states. Epidemiology and Infection 2013; 141: 1625–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauri AM, Uphoff H, Gawrich S. The burden of acute gastrointestinal illness in Hesse – a telephone survey [in German]. Das Gesundheitswesen 2011; 73: 78–84. [DOI] [PubMed] [Google Scholar]
- 21.Kist MM, et al. MIQ 09: Gatrointestinal Infections. Quality Standards in Microbiological Dagnostics, 2nd edn [in German]. Urban & Fischer Verlag/Elsevier, 2013.
- 22.Vose D. Risk Analysis: A Quantitative Guide, 3rd edn. West Sussex, England: John Wiley & Sons, 2008. [Google Scholar]
- 23.Havelaar AH, et al. Disease burden in the netherlands due to infections with shiga toxin-producing Escherichia coli O157. Epidemiology and Infection 2004; 132: 467–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alpers K, et al. Sorbitol-fermenting enterohaemorrhagic Escherichia coli O157:H- causes another outbreak of haemolytic uraemic syndrome in children. Epidemiology and Infection 2009; 137: 389–395. [DOI] [PubMed] [Google Scholar]
- 25.Werber D, et al. A multistate outbreak of shiga toxin-producing Escherichia coli O26:H11 infections in Germany, detected by molecular subtyping surveillance. Journal of Infectious Diseases 2002; 186: 419–422. [DOI] [PubMed] [Google Scholar]
- 26.European Centre for Disease Prevention and Control. Surveillance of six priority food- and waterborne diseases in the eu/eea 2006–2009. Stockholm: ECDC, 2013.
- 27.European Centre for Disease Prevention and Control. Surveillance of seven priority food- and waterborne diseases in the EU/EEA 2010–2012. Stockholm: ECDC, 2015.
- 28.Werber D, et al. Shiga toxin-producing Escherichia coli serogroups in food and patients, germany. Emerging Infectious Diseases 2008; 14: 1803–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiska DL, Riddell SW. Counterpoint: should all stools be screened for shiga toxin-producing Escherichia coli? Journal of Clinical Microbiology 2011; 49: 2394–2397. [PubMed] [Google Scholar]
- 30.Marcon MJ. Point: Should all stools be screened for shiga toxin-producing Escherichia coli? Journal of Clinical Microbiology 2011; 49: 2390–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould LH, et al. Increased recognition of non-O157 shiga toxin-producing Escherichia coli infections in the united states during 2000–2010: epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathogens and Disease 2013; 10: 453–460. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen S, et al. Desperately seeking diarrhoea: outbreak of haemolytic uraemic syndrome caused by emerging sorbitol-fermenting shiga toxin-producing Escherichia coli O157:H–, Germany, 2009. Journal of Zoonoses in Public Health 2011; 58: 567–572. [DOI] [PubMed] [Google Scholar]
- 33.Werber D, et al. Looking for tips to find icebergs – surveillance of haemolytic uraemic syndrome to detect outbreaks of shiga toxin-producing E. coli infection. Eurosurveillance 2008; 13. [PubMed] [Google Scholar]
- 34.Mahon BE, et al. Hemolytic uremic syndrome surveillance to monitor trends in infection with Escherichia coli O157:H7 and other shiga toxin-producing E. coli. Emerging Infectious Diseases 1997; 3: 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268816001436.
click here to view supplementary material