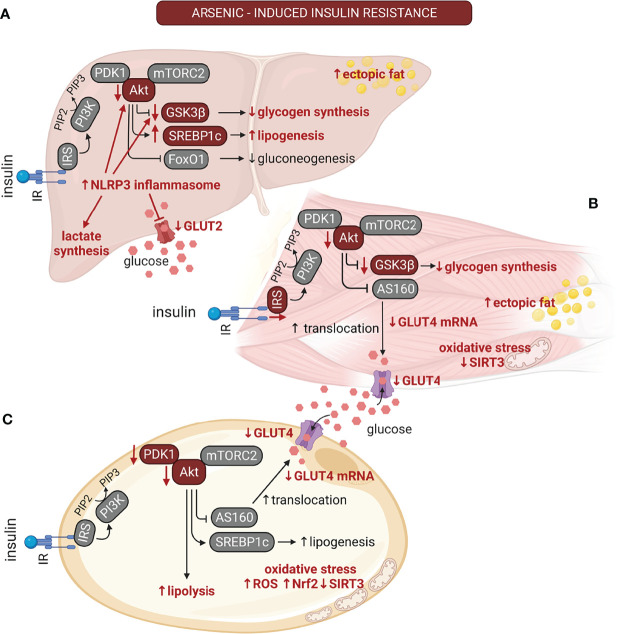
Figure 2.
The multiple effects of arsenic on insulin signaling are tissue-dependent. The alterations induced by arsenic exposure on the insulin signaling pathway are depicted with red font. Arsenic induces insulin resistance in classical insulin targeted organs. (A) In liver, arsenic reduces GLUT2 protein levels and reduces glycogen synthesis due to NLR family pyrin domain containing 3 (NLRP3) inflammasome activation. This also results in reduction in the phosphorylation of Akt and glycogen synthase kinase 3β (GSK3β). Also, it promotes liver lipogenesis and the development of non-alcoholic fatty liver disease (NAFLD). (B) In muscle, arsenic inhibits GLUT4 expression. It also reduces insulin signaling by inhibiting IRS-1 and Akt phosphorylation, reducing insulin-stimulated GLUT4 translocation and decreased glycogen levels, thus contributing to hyperglycemia in the postprandial state. Arsenic can also promote ectopic fat deposition in muscle. (C) In adipose tissue, this pollutant induces adipocyte hypertrophy, impaired adipogenesis, and suppression of mitochondrial biogenesis. Arsenic stimulates lipolysis, and oxidative stress due to reduced SIRT3 activity. On the other hand, it inhibits GLUT4 expression and insulin-stimulated GLUT4 translocation by inhibition of Akt phosphorylation. Akt, protein kinase B; AS160, Akt substrate 160 kDa; FoxO1, forkhead-box-O1; GLUT4, glucose transporter 4; GSK3β, glycogen synthase kinase 3β; IR, insulin receptor; IRS, insulin receptor substrate; mTORC2, mammalian target of rapamycin complex 2; NLRP3, NLR family pyrin domain containing 3; Nrf2, nuclear factor erythroid 2-related factor 2; PDK1, Phosphoinositide-dependent kinase-1; PI3K, phosphatidyl inositol 3 kinase; PIP2, phosphatidyl inositol 4;5-biphosphate; PIP3, phosphatidyl inositol 3;4;5 triphosphate; ROS, reactive oxygen species; SIRT3, sirtuin-3; SREBP1c, sterol regulatory element-binding transcription factor 1c. Created with BioRender.com.