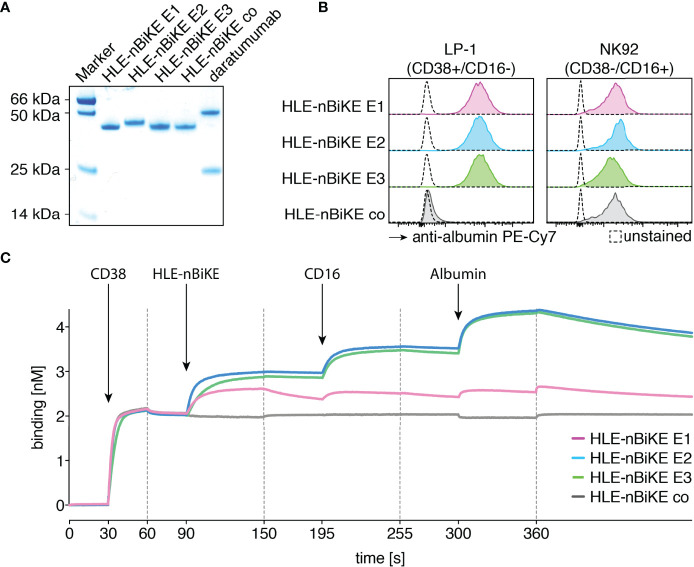
Figure 2.
Purified CD38-specific HLE-nano-BiKEs bind specifically and simultaneously to myeloma cells, NK cells, and albumin. (A) Purified HLE-nano-BiKEs and daratumumab (1 µg per lane) were size fractionated by SDS-PAGE under reducing conditions and visualized by Coomassie staining. (B) LP-1 luc myeloma cells (left panel) and NK92 hCD16 cells (right panel) were incubated with CD38-specific HLE-nano-BiKE E1, HLE-nano-BiKE E2, HLE-nano-BiKE E3, or an isotype control (HLE-nano-BiKE co). Bound HLE-nano-BiKEs were detected by flow cytometry using biotinylated human albumin and PE-Cy7-conjugated streptavidin by flow cytometry. Control stainings (unstained, dashed line) were performed with albumin and PE-Cy7-conjugated streptavidin alone. Results are representative of three similar experiments. (C) BLI analyses of the sequential binding of HLE-nano-BiKEs, CD16 and albumin to immobilized CD38. The biotinylated recombinant ectodomain of human CD38 was bound to a streptavidin-coated BLI sensor (30-90s). HLE-nano-BiKEs, recombinant ectodomain of human CD16, and human albumin were added at the times indicated. In each case binding was measured for 60s with a subsequent 60s dissociation phase. Arrows indicate the time point of protein addition; dashed lines indicate the start of the dissociation phase.