SUMMARY
The association between herpes zoster and subsequent cancer risk is still unclear. Consequently, doubts remain regarding the need for investigation of herpes patients for co-existing or subsequent malignancy. This is a retrospective cohort study comparing cancer risk in patients after herpes zoster and age-/sex-matched non-herpes zoster patients, in a primary care-based continuous morbidity database. We tested for interaction by gender, age, diabetes, HRT use or antiviral therapy. Analyses were repeated for patients with and without herpes simplex. The hazard ratio (HR) comparing cancer risk in herpes zoster vs. control patients was significant in all women, women aged > 65 years and subgroups of breast and colorectal cancer (HRs 1·60, 1·82, 2·14, 2·19, respectively). For men, a significant association was found for haematological cancers (HR 2·92). No associations were found with herpes simplex. No interaction was identified with antiviral therapy, diabetes or HRT treatment. We concluded that there was a moderate significant association between herpes zoster and subsequent cancer risk in women aged > 65 years, without any influence of antiviral therapy. No association was found with herpes simplex. There is insufficient reason for extensively testing older patients with herpes zoster or herpes simplex for the presence of occult cancer.
Key words: Herpes zoster, malignancy, retrospective cohort study, survival analysis
INTRODUCTION
Herpes zoster usually emerges as anti-varicella zoster virus antibodies and T cell responses wane with age [1]. Given that the incidence of most malignancies is also strongly related to ageing, there have been several attempts to try to determine whether the emergence of zoster could be used as a marker of increased risk of cancer and a signal to actively seek occult malignancy. Studies so far have been contradictory [2–7]. Using data of 311 000 patient-years, we previously demonstrated an increased risk of malignancy in women aged > 65 years [3], suggesting that herpes zoster infection may be a marker of a cancer-susceptible immune system.
It has been difficult to provide a definitive answer regarding the association of zoster with malignancy in community settings, due to the limited size of available datasets. Using our general practice (GP)-based morbidity register we analysed data relating to 1474993 patient-years. We used this dataset to re-examine cancer risk in patients with and without previous herpes zoster, controlling for age and gender. We also tested whether any association with malignancy is specific for herpes zoster or also present for other herpes virus infections. If such relation is present, this can refer to either a direct association between herpes and malignancy or to the effect of treatment given. We therefore tested whether there would be any effect modification by antiviral therapy.
PATIENTS AND METHODS
Patients
Data were obtained from Intego, an ongoing general practice-based morbidity registration network based in the Department of General Practice at the Catholic University of Leuven [3]. Ninety GPs evenly spread over Flanders, Belgium collaborated in the Intego project. Before their data are included, their registration performance is audited and only data from the practices with the best recording performance (less than 50% of the applicants) are included in the database. The Intego GPs are continuously and prospectively registering all new diagnoses together with new drug prescriptions, laboratory test results, and some background information (including gender and year of birth), using keywords linked to codes. Using specially framed extraction software, new data are collected from the GPs’ computers and entered into a central database. Registered data are continuously updated and historically accumulated for each patient. New diagnoses are classified according to ICPC-2 (International Classification of Primary Care) [8], a classification system for morbidity in GP accepted and used worldwide. Medications are classified according to the WHO's Anatomical Therapeutic Chemical (ATC) classification system [9]. The Intego population represents the Flemish population with respect to age and gender distribution [10].
For this study, diagnoses and prescribed medications recorded during the years 1994–2008 were used, together with their dates. They cover information on 215 251 different patients and 1 474 993 patient-years. This includes almost 2% of the Flemish population.
Definitions and codes
Within the Intego network, no predefined criteria were used for entering a diagnosis. The registering GPs used their own clinical skills and used additional tests as considered appropriate. In complex medical conditions, or in case of a patient directly consulting a specialist, inclusion is based on specialist diagnoses that are reported to the GP by letter. This information is considered to be complete, with some uncertainties in ophthalmology, gynaecology and paediatrics.
Since herpes zoster is a clinical diagnosis, no other methods of confirmation of this diagnosis were used. Our incidence rate (3·3/1000 person-years) was consistent with other studies (between 3·2 and 4·2 cases/1000 person-years) [11]. We separately tested for a possible inclusion of prevalent cases by comparing the incidence in the first registration year with subsequent years and found no differences [12]. The same reasoning applied for herpes simplex. For herpes simplex, diagnoses of herpes labialis (type 1) and herpes simplex (type 2) were combined.
A different method was used to ascertain incident cases of malignancy. We selected a random sample of patients with a diagnosis of malignancy per practice and asked the physicians for the tests on which each diagnosis was based. We received information from 48 practices and 463 patients. Two patients could not be retrieved by the GP, the diagnosis was confirmed histologically in 73%, at least cytologically in an additional 8%, and based on magnetic resonance imaging (MRI), computerized axial tomography (CAT) scan or endoscopy in 10%. Four percent was based on classical radiology and 2% were clinical diagnoses. Of the 14 remaining cases no information on method of diagnosis was available, but 12 of the cases were diagnosed during a hospital stay. The Intego cancer incidence (3·63/1000 patient-years) was largely similar to a local cancer registry [13] (4·25/1000 person-years over 1996–2005). In accordance with the rules in most cancer registries, basal cell carcinoma was not considered as a malignancy.
Antiviral drug was defined as a drug classified under ATC code J05AB [9].
Retrospective cohort study
Patients with a diagnosis of herpes zoster between 1 January 1994 and 31 December 2008 were included, with the date of diagnosis as the baseline date. Controls without any diagnosis of herpes zoster were randomly matched to the cases by year of birth and gender. We aimed to match six controls to each case, although for some cases only four or five controls were available. The date of diagnosis of the index patient was assigned to the no-herpes zoster patients as the baseline date for the study. To prevent double use of control patients when more than one patient from the same sex and year of birth was diagnosed with the index disease, such dates were randomly assigned to all control patients from the same sex and birth year. Similar matching was done for all cases of herpes simplex.
In both groups, we excluded those who were diagnosed with malignancy before or at the date of inclusion. Both groups of patients (with and without the index disease) were followed for an emerging first diagnosis of malignancy. Follow-up of patients lasted until the end of the registration period, or earlier in case of emergence of cancer or a patient leaving the Intego patient population, e.g. by relocation or death.
Analysis
All analyses were performed separately for men and women.
Survival analyses were performed to take account of the censored nature of the data, For patients without any diagnosis of cancer, due to privacy regulations, only the last year of contact could be obtained, not the exact date. Hence, for those patients, the last date of contact was set to 30 June of that year.
Kaplan–Meier curves were calculated for time to diagnosis of cancer for patients with and without herpes zoster/herpes simplex. In order to take account of the matched nature of the data, a weighted Kaplan–Meier curve for matched data was constructed [14].
In addition, a Cox regression was performed whereby the matched nature of the data was accounted for by using the robust sandwich estimate of Lin & Wei [15] for the covariance matrix. All analyses were also performed for each gender separately, and adjusted for age. Hazard ratios (HR) and their 95% confidence intervals (CI) were calculated and differences tested by means of a Wald test, using the robust sandwich estimator for the standard error [13]. The proportional hazards assumption was assessed by visual inspection of the Kaplan–Meier curves and was found to be satisfied. We additionally performed further checks of the appropriateness of the proportional hazards assumptions using the methods described by Lin et al. [16] using cumulative sums of martingale residuals. Although small deviations were found for some of our analyses, generally the assumption was sufficiently satisfied.
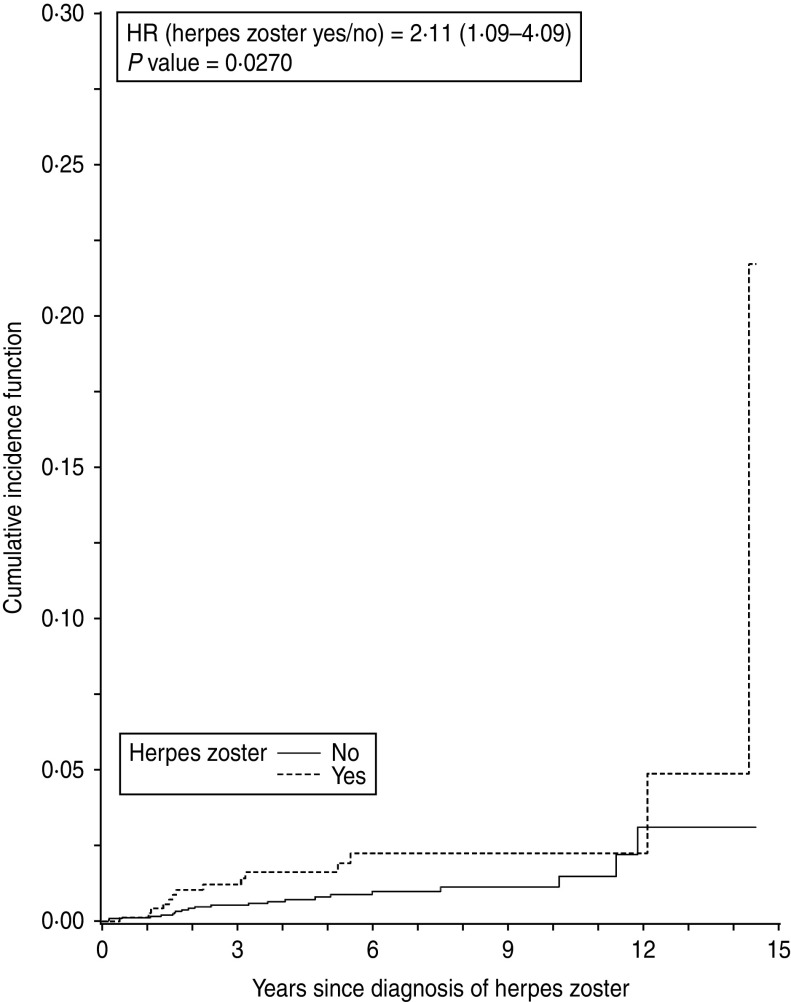
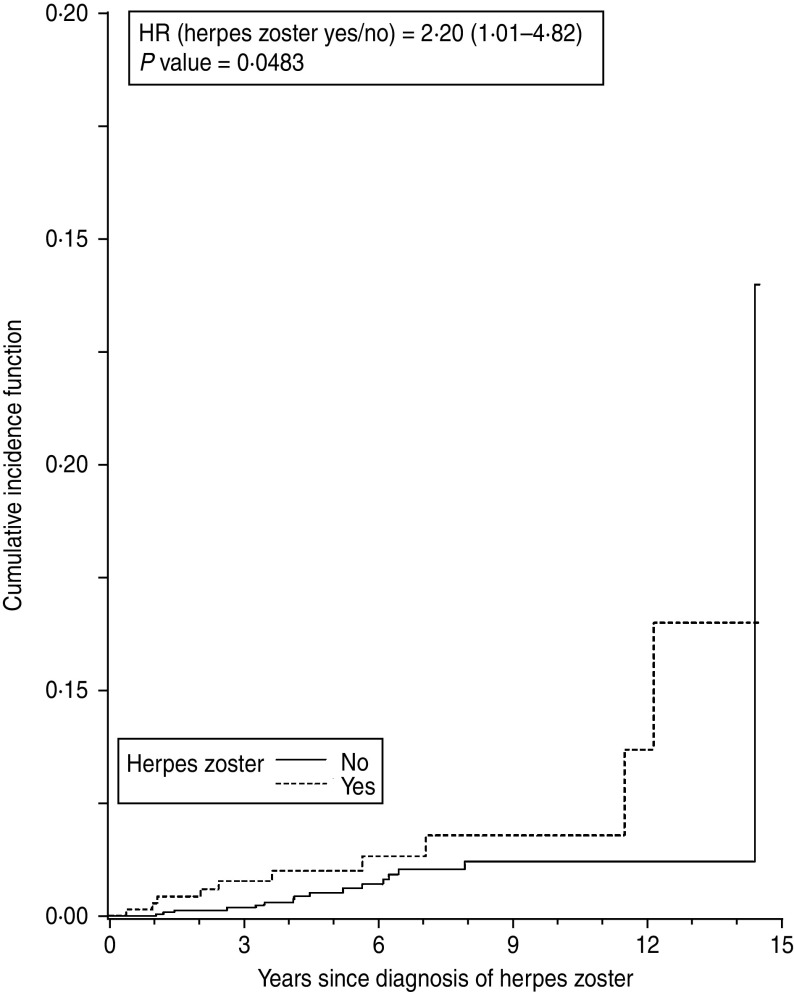
Subgroup analyses were defined a priori and performed for age groups, antiviral drug use after the herpes diagnosis, hormone replacement therapy (HRT) use (in women only), and comorbid diabetes at baseline. Due to the expected low number of cases of malignancies and herpes zoster cases aged ⩽65 years, the latter analyses were restricted to patients aged > 65 only. Cancer-specific incidence of cancer according to herpes zoster was estimated using cumulative incidence functions (Figs 3, 4).
Fig. 3.
Time to emergence of breast cancer after herpes zoster or matching date in women aged > 65 years.
Fig. 4.
Time to emergence of colorectal cancer after herpes zoster or matching date in women aged > 65 years.
We formally tested for interaction between herpes zoster and antiviral treatment, HRT use and diabetes. Statistical tests were two-sided and performed at a significance level of 5%. Due to the exploratory nature of the study, no adjustments were made to the significance levels to account for multiple testing [17]. All analyses were performed using SAS software, version 9.2 [18].
Ethical considerations
Before sending the data to the central database, patient identification information was encrypted in each practice using a one-way encryption algorithm. As a result, only the registering GP is able to identify which patient a certain code belongs to. Because of the large number of patients involved, it is impossible to individually inform each patient concerned. However, according to the national privacy law patients are informed about the registration through posters displayed in the waiting rooms of the GPs. The Intego procedures have been approved by the ethical review board of the Medical School of the Catholic University of Leuven (no. ML1723).
RESULTS
Patient characteristics
In total, 2635 women and 2186 men had a diagnosis of herpes zoster, and were matched with 12 827 and 10 594 controls, respectively. Following a diagnosis of herpes zoster, 75 (2·8%) women and 78 (3·6%) men had a diagnosis of cancer compared to 159 (1·2%) female and 216 (2·0%) male controls (Table 1). Median time from herpes zoster to cancer diagnosis was 3·00 years (range 1·34–6·02 years).
Table 1.
Flowchart of the study population
| Intego population total (N = 215 251) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Men (n = 104 894) | Women (n = 110 357) | |||||||
| Herpes zoster (herpes simplex) | Yes | No | Yes | No | ||||
| All patients | 2186 (932) | 102 708(103 962) | 2635 (1561) | 107 722(108 796) | ||||
| Included in the study* | 2186 (932) | 10 594(4599) | 2635 (1561) | 12 827(7686) | ||||
| Subsequent malignancy | Yes | No | Yes | No | Yes | No | Yes | No |
| 78 (14) | 2108 (918) | 216 (56) | 10 378 (4 543) | 75 (20) | 2560 (1 541) | 159 (68) | 12 668 (7 618) | |
All herpes zoster (herpes simplex) patients and a random sample of matched controls.
A total of 1561 women and 932 men had a first diagnosis of herpes simplex, to which 7686 female and 4599 male controls were matched, respectively. Following a diagnosis of herpes simplex, 20 (1·3%) women and 14 (1·5%) men had a diagnosis of cancer compared to 68 (0·9%) female and 56 (1·2%) male controls (Table 1). The median time from herpes simplex to cancer diagnosis was 4·09 years (range 1·77–8·63 years).
The number of patients with herpes zoster treated with antiviral drugs was 1239 (57%) and 1534 (58%) in men and women, respectively. For patients with herpes simplex the numbers were 218 (23%) and 416 (27%).
Demographic characteristics of all patients are listed in Table 2.
Table 2.
Descriptive characteristics of the study population
| Patients with herpes zoster (n = 4821) | Patients without herpes zoster (n = 23 421) | Patients with herpes simplex (n = 2493) | Patients without herpes simplex (n = 12 285) | |
|---|---|---|---|---|
| Number of patients with malignancy (%) | 153 (3·2) | 375 (1·6) | 34 (1·4) | 124 (1·0) |
| Mean age at baseline, years (s.d.) | 47·5 (23) | 46·8 (23) | 35·9 (21) | 35·6 (20) |
| Sex, n (%) | ||||
| Men | 2186 (45) | 10 594 (45) | 932 (37) | 4599 (37) |
| Women | 2635 (55) | 12 827 (55) | 1561 (63) | 7686 (63) |
| Age group at baseline, years (%) | ||||
| 0–50 | 2248 (47) | 11 176 (48) | 1872 (75) | 9299 (76) |
| 51–65 | 1172 (24) | 5697 (24) | 382 (15) | 1858 (15) |
| >65 | 1401 (29) | 6548 (28) | 239 (10) | 1128 (9) |
s.d., Standard deviation.
Herpes zoster and subsequent cancer risk
Crude data analysis
The overall HR for both men and women together, adjusted for age (spline) and gender was 1·37 (95% CI 1·14–1·66, P = 0·001), indicating a significantly higher risk of subsequent malignancy after herpes zoster compared to controls. There was no interaction between herpes zoster and gender (P = 0·12).
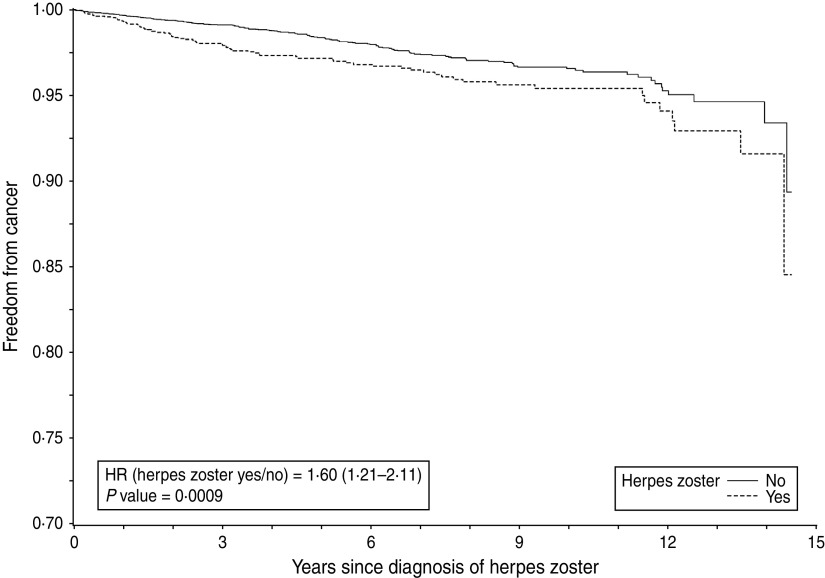
HRs by gender and age group are summarized in Table 3. The estimated HR for the comparison between cases and controls in women was 1·60 (95% CI 1·21–2·11, P< 0·001). The Kaplan–Meier curve is presented in Figure 1. The HR after 1 year of follow-up was 2·11 (95% CI 1·15–3·87).
Table 3.
Association between herpes zoster and subsequent malignancy: estimated age-adjusted hazard ratios according to age group, site and gender
| Age (no. of events in men + women) | Site | Men HR (95% CI) | Women HR (95% CI) |
|---|---|---|---|
| All (294 + 234) | All | 1·09 (0·91–1·54) | 1·60 (1·21–2·11) |
| ⩽50 (9 + 38) | All | 0·87 (0·20–3·72) | 1·16 (0·56–2·41) |
| 51–65 (90 + 75) | All | 1·07 (0·67–1·73) | 1·53 (0·93–2·50) |
| >65 (195 + 121) | All | 1·28 (0·93–1·75) | 1·82 (1·25–2·66) |
| >65 (50 + 5) | Lung | 1·19 (0·63–2·25) | 0·92 (0·11–7·94) |
| >65 (53 + 0) | Prostate | 1·10 0·59–2·05) | † |
| >65 (0 + 37) | Breast | † | 2·14 (1·11–4·12) |
| >65 (23 + 26) | Colorectal | 1·84 (0·78–4·34) | 2·19 (1·00–4·80) |
| >65 (19 + 14) | Haematological | 2·92 (1·20–7·08) | 0·58 (0·13–2·62) |
HR, Hazard ratio; CI, confidence interval.
No cases of malignancy were recorded.
Fig. 1.
Time to emergence of malignancy after herpes zoster or matching date in all women.
The estimated HR for the comparison between cases and controls in men was 1·09 (95% CI 0·91–1·54, P = 0·20), thus yielding no statistical evidence of an association between herpes zoster and subsequent malignancy in men.
Subgroup analyses according to age and cancer localization
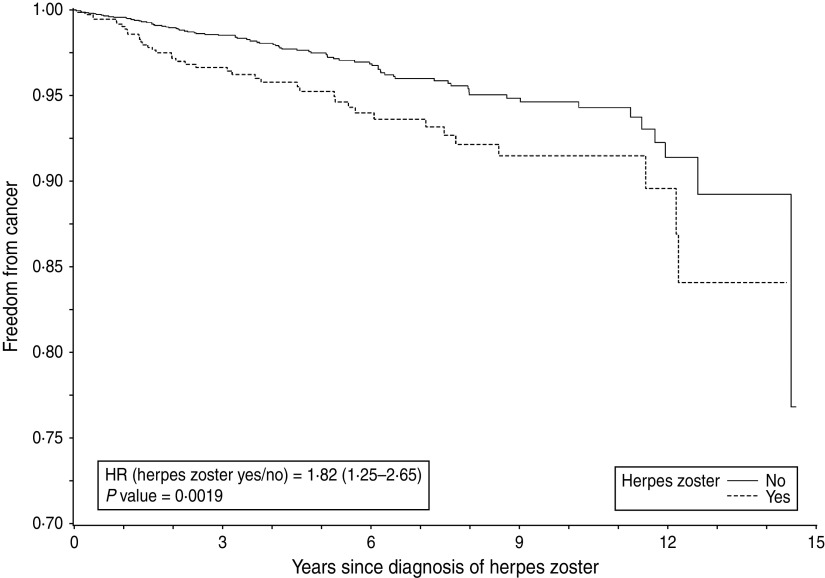
Subgroup analyses in women revealed that the association between herpes zoster and a subsequent cancer diagnosis was only relevant in the older age groups, especially for breast and colorectal cancer. For individuals aged > 65 years, the HR for any cancer was 1·82 (95% CI 1·25–2·66, P = 0·002) (Fig. 2). A statistically significant association was also found between herpes zoster and diagnosis of colorectal cancer (HR 2·19, 95% CI 1·00–4·80, P = 0·05) and breast cancer (HR 2·14, 95% CI 1·11–4·12, P = 0·02). No evidence for an association between herpes zoster and lung or haematological cancer could be found (Table 3). Cancer-specific cumulative incidence functions are presented in Figures 3 and 4.
Fig. 2.
Time to emergence of malignancy after herpes zoster or matching date in women aged > 65 years.
For men, subgroup analyses for age and cancer localization showed a statistically significant association for haematological cancer (HR 2·92, 95% CI 1·20–7·08, P = 0·02) only (Table 3).
In patients aged > 50 years, the significant associations were exactly the same, albeit with slightly lower HRs (data not shown).
The effect of possible effect modifiers
Formal analyses in patients aged > 65 years showed no statistical interaction between antiviral drug use, diabetes or HRT use and herpes zoster.
Herpes simplex and subsequent cancer risk
Crude data analysis
The HR for the comparison between women or men with and without herpes simplex was 1·06 (95% CI 0·64–1·74) or 0·91 (95% CI 0·49–1·70), suggesting absence of association (Table 4). A Kaplan–Meier curve is presented in Figure 5.
Table 4.
Association between herpes simplex and subsequent malignancy: estimated age-adjusted hazard ratios according to age group, site and gender
| Age (no. of events in men + women) | Site | Men HR (95% CI) | Women HR (95% CI) |
|---|---|---|---|
| All (70 + 88) | All | 0·91 (0·49–1·70) | 1·06 (0·64–1·74) |
| ⩽50 (5 + 32) | All | 0·80 (0·08–8·08) | 1·17 (0·52–2·63) |
| 51–65 (30 + 28) | All | 1·01 (0·41–2·46) | 0·85 (0·33–2·18) |
| >65 (35 + 28) | All | 0·68 (0·35–2·11) | 1·18 (0·49–2·87) |
| >65 (11 + 0) | Lung | 0·84 (0·18–3·97) | † |
| >65 (9 + 0) | Prostate | ‡ | † |
| >65 (0 + 8) | Breast | † | 1·18 (0·24–5·82) |
| >65 (1 + 7) | Colorectal | † | 1·42 (0·24–8·54) |
| >65 (6 + 7) | Haematological | 2·06 (0·37–11·56) | 1·48 (0·29–7·44) |
HR, Hazard ratio; CI, confidence interval.
No cases of malignancy were recorded in at least one of the comparison groups.
Only one case of malignancy was recorded in at least one of the comparison groups.
Fig. 5.
Time to emergence of malignancy after herpes simplex or matching date in all female patients.
Subgroup analyses according to age and cancer localization
Subgroup analyses revealed no significant associations for any of the subgroups. Care should be taken with the analyses by cancer type due to the low number of events for patients with herpes simplex (Table 4).
DISCUSSION
Summary of main findings and relationship with the existing literature:
Our results indicate a moderate association between herpes zoster and subsequent cancer risk in women aged > 65 years. In this group, 102 (95% CI 51–333) cases of herpes zoster are related to one additional malignancy. The association was largely similar during the first year, with one additional malignancy for 282 cases of herpes zoster. This association was most prominent for breast and colorectal cancer. In men a significant association was absent for all malignancies except haematological cancers. No associations could be found in men or women between herpes simplex and cancer. These results confirm and enlarge the initial results reported in our previous study based on the first 7 years of registration [3].
Our results are largely comparable to the results of a recently published, similar, larger and UK-based study [6], although in that study the results for men only were also significant. Another recent study from Taiwan [7] resulted in significantly positive HRs for men and women combined for the first 2 years only. No gender-stratified results were reported. No relationship could be identified in patients aged ⩽50 years. However, such a relationship would be difficult to prove, due to the low number of patients with herpes zoster and cancer in these age groups, even in a large cohort. As a sensitivity analysis, we also tested the associations in the population aged > 50 years. The results were largely similar as for patients aged > 65 years, although with slightly lower HRs.
The restriction of significant results for women only is less easy to explain. However, the 95% CIs of HRs for men and women overlap, so a power problem may be involved.
The association with haematological cancer in males could result from HIV. However, the number of patients with HIV infection in the whole database is only 13. An intermediate effect of sex hormones could be hypothesized, at least for breast cancer; the influence of HRT in the emergence of breast cancer has extensively been described [19, 20]. In a post-hoc sensitivity analysis, we compared the association between herpes zoster and subsequent breast cancer risk in patients with and without HRT use and found similar HRs (2·27 and 2·19).
As the association between herpes zoster and subsequent cancer diagnosis was not larger during the first year of follow-up, the results do not support an important role of acute herpes zoster as a marker of an undiagnosed malignancy as regularly discussed since 1955 [3, 5–7, 21, 22].
Possible mechanisms
We tested for possible effect modification by antiviral treatment and diabetes on the association between herpes zoster and subsequent cancer risk and found none. This finding on antiviral treatment is also congruent with previous studies indicating absence of a carcinogenic effect of antivirals [23].
As some cancers are known to be present in a pre-clinical and undetectable form for ⩾10 years before diagnosis [24, 25], herpes zoster could be an early manifestation of the impairment of the immune system provoked by the (occult) malignancy. It seems impossible, however, to test this hypothesis.
Alternatively, it is possible that herpes zoster virus provokes antigenic stimulation or changes tissue antigen with some effect on the development of malignancy. Such reasoning is supported by the finding of different types of malignancies (lymphoma, pseudolymphoma, angiosarcoma, Kaposi's sarcoma) at the same site as a previous herpes zoster infection [26, 27].
Finally, both reactivation of the virus and immunological surveillance against malignancy [28, 29] are related to cellular immunity (CD4+ and CD8+ T cells and natural killer cells). Any dysfunction of such a mechanism can therefore result in the emergence of both herpes zoster and malignancy. In some cases herpes zoster would emerge first and in other cases malignancy.
Finally, possible confounders we do not know or expect, and therefore cannot control, or even an indicator effect of herpes zoster for another exposure factor unknown to us could influence our results.
The fact that no association was found between a clinical episode of herpes simplex and subsequent cancer risk suggests a virus-specific effect of herpes zoster and not herpes simplex, probably interacting with a more serious degree of immunosenescence. Herpes simplex virus 1 and 2 and the varicella virus are all alpha-herpes viruses, but genetically they are very different. However, failure to find an association may result from either absence of such an association or low power. Contrary to herpes zoster, herpes simplex will not always trigger a contact with a physician. An unknown proportion of patients will accept this condition as something that will come and go without any need to seek professional medical care. For our analysis, this would result in a bias towards a null effect. Even if an underlying immunological mechanism were present, it would be difficult to detect and relate to subsequent cancer occurrence.
Weaknesses
It should be borne in mind that the censoring in this database is not necessarily non-informative: diagnosing one disease (e.g. herpes zoster) could easily stimulate increased searching and diagnosing of other diseases (e.g. malignancy). Additionally, healthcare-seeking behaviour of patients seen after herpes zoster may be different from individuals without herpes. Therefore, a diagnosis of malignancy could be made earlier in herpes patients and inflate the HR. However, the presence of such indication bias would result in a biased association during the first months after the first diagnosis only. In our study, although there was an association between herpes zoster and subsequent malignancy during the first year in women aged > 65 years, the HR of 2·11 for the first year was even lower than the HR in following years.
The percentages of non-cancer patients censored during follow-up and before study end were 54% for patients without and 32% for patients after herpes zoster. In patients without malignancy, the mean follow-up time was also shorter in the group without herpes zoster compared to the zoster group. It is possible that patients die faster in the non-zoster group and therefore have less time available to develop a malignancy, but the opposite is also possible: these patients may be very healthy and not have visited a physician for many years. Because date of death is not available, we cannot test these hypotheses.
In a study testing for a large number of possible associations, we also have to deal with a multi-comparison problem. As this is an observational study and in accordance with Rothman [17] and many others, we decided not to formally adjust our P values by using a Bonferroni or other method. However, for all cancers and for breast cancer in women aged > 65 years, we found P values of 0·001 and 0·002, respectively. Moreover, for all significant associations in patients aged > 65 years the measures of effect (HRs) were very reassuring.
The mean age of herpes zoster patients tends to be older compared to herpes simplex patients. As a result of the matching of controls by age this is also the case for their respective control groups. As age is the main risk factor for malignancy, it is normal that cancer incidence is higher in the zoster control group than in the simplex control group.
Diagnosis of herpes is a clinical diagnosis and therefore susceptible to measurement bias. It is encouraging, however, that our rates of herpes zoster are comparable to those reported in the literature.
Strengths
Our results are based on routinely collected data without any selection apart from the decision of a patient to present his complaints to a physician or not, and thus originate from real life. The analyses include the major cancer locations with sufficient power and we were able to test for effect modification by relevant comorbidities or drug treatment. Follow-up ranges up to a maximum of 15 years.
Implications for future research
The presence of additional comorbid diseases or chronic use of medication at baseline, or even the severity of herpes zoster or the frequency of contact with children (and therefore the likelihood of re-exposure to the virus) might influence the associations we described. We have no realistic hypothesis, however, about the nature or mechanisms of such an effect. Testing a large number of comorbid diseases for a possible confounding effect would be possible, even within the framework of the Intego database. It would, however, enormously increase the multi-comparison problem, decrease statistical power and easily degenerate into a fishing expedition. Additionally this report may stimulate researchers from other disciplines to start research into the immunological basis of our findings.
Implications for clinical practice
As a general conclusion, at present we see no reason for extensively testing for occult cancer in patients with a clinical episode of herpes zoster or herpes simplex. However, our results highlight the need for clinicians to carefully follow the current cancer screening and early diagnosis guidelines in women after herpes zoster.
Supplementary Material
Supplementary information supplied by authors.
ACKNOWLEDGEMENTS
This work would not have been possible without the collaboration of all general practitioners of the Intego network. The Intego network is funded by the Flemish Health Ministry. There was no additional funding for this study.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268813001702.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Arvin A. Aging, immunity, and the varicella-zoster virus. New England Journal of Medicine 2005; 352: 2267–2267. [DOI] [PubMed] [Google Scholar]
- 2.Smith JB, Fenske NA. Herpes zoster and internal malignancy. Southern Medical Journal 1995; 88: 1089–1092. [DOI] [PubMed] [Google Scholar]
- 3.Buntinx F, et al. Is herpes zoster a marker for occult or subsequent malignancy? British Journal of General Practice 2005; 55: 102–107. [PMC free article] [PubMed] [Google Scholar]
- 4.Fueyo MA, Lookingbill DP. Herpes zoster and occult malignancy. Journal of the American Academy of Dermatology 1984; 11: 480–482. [DOI] [PubMed] [Google Scholar]
- 5.Ragozzino MW, et al. Risk of cancer after herpes zoster: a population-based study. New England Journal of Medicine 1982; 307: 393–397. [DOI] [PubMed] [Google Scholar]
- 6.Cotton SJ, et al. The risk of a subsequent cancer diagnosis after herpes zoster infection: primary care database study. British Journal of Cancer 2013; 108: 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hui-Fen C, Chen BK, Yang CY. Herpes zoster and subsequent risk of cancer: a population-based study. Journal of Epidemiology. doi: 10.2188/jea.JE20120155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okkes IM, et al. The March 2002 update of the electronic version of ICPC-2. A step forward to the use of ICD-10 as a nomenclature and a terminology for ICPC-2. Family Practice 2002; 19: 543–546. [DOI] [PubMed] [Google Scholar]
- 9.WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2010. (http://www.whocc.no/atc_ddd_index/). Accessed 18 July 2013.
- 10.Intego. www.intego.be. Accessed 1 April 2013.
- 11.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the advisory committee on immunization practices. Morbidity and Mortality Weekly Report 2008; 57: 1–30. [PubMed] [Google Scholar]
- 12.Truyers C, et al. Intego in daily practice: Incidence of herpes zoster. Huisarts Nu 2005; 34: 260–262. [Google Scholar]
- 13.Lousbergh D, et al. Ten years of cancer in the Belgian province of Limburg (1996–2005). Hasselt, LIKAS, 2007.
- 14.Galimberti S, Sasieni P, Valsecchi MG. A weighted Kaplan-Meier estimator for matched data with application to the comparison of chemotherapy and bone-marrow transplant in leukaemia. Statistics in Medicine 2002; 21: 3847–3864 [DOI] [PubMed] [Google Scholar]
- 15.Lin DY, Wei LJ. The robust inference for the proportional hazards model. Journal of the American Statistical Association 1989; 84: 1074–1078 [Google Scholar]
- 16.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika 1993; 80: 557–572. [Google Scholar]
- 17.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1: 43–46. [PubMed] [Google Scholar]
- 18.SAS Institute Inc. SAS software, version 9.2 of the SAS System for Windows. SAS Institute Inc., Cary, NC, USA: 2002. [Google Scholar]
- 19.Vankrunkelsven P, et al. Reduction in hormone replacement therapy and declining breast cancer incidence in the Belgian province of Limburg. Breast Cancer Research and Treatment 2009; 118: 425–432. [DOI] [PubMed] [Google Scholar]
- 20.Kumle M. Declining breast cancer incidence and decreased HRT use. Lancet 2008; 372: 608–610. [DOI] [PubMed] [Google Scholar]
- 21.Wyburn-Mason R. Malignant change arising in tissue affected by herpes. British Medical Journal 1955; 2: 1106–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serraino D, et al. Socio-economic indicators, infectious diseases and Hodgkin's disease. International Journal of Cancer 1991; 47: 352–357. [DOI] [PubMed] [Google Scholar]
- 23.Tucker WE Jr., et al. Preclinical toxicology studies with acyclovir: carcinogenicity bioassays and chronic toxicity tests. Fundamental and Applied Toxicology 1983; 3: 579–586. [DOI] [PubMed] [Google Scholar]
- 24.Gramenzi A, et al. Medical history and the risk of multiple myeloma. British Journal of Cancer 1991; 63: 769–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Vecchia C, Negri E, Franceschi S. Medical history and the risk of non-Hodgkin lymphomas. Cancer Epidemiology, Biomarkers and Prevention 1992; 1: 553–556. [PubMed] [Google Scholar]
- 26.Wolff HH, Wendt V, Winzer M. Cutaneous pseudolymphoma at the site of prior herpes zoster eruption. Archives of Dermatological Research 1987; 279: S52–S54. [DOI] [PubMed] [Google Scholar]
- 27.Mishra D, Raji MA. Squamous cell carcinoma occurring at site of prior herpes zoster of the scalp: case report of Marjolin ulcer. Melanoma Research 2003; 13: 635–639. [DOI] [PubMed] [Google Scholar]
- 28.Konjevic G, et al. The difference in NK-cell activity between patients with non-Hodgkin's lymphomas and Hodgkin's disease. British Journal of Haematology 1999; 104: 144–151. [DOI] [PubMed] [Google Scholar]
- 29.Yang CM, et al. Natural killer cell deficiency associated with Hodgkin's lymphoma. Journal of the Formosan Medical Association 2002; 101: 73–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information supplied by authors.
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268813001702.
click here to view supplementary material