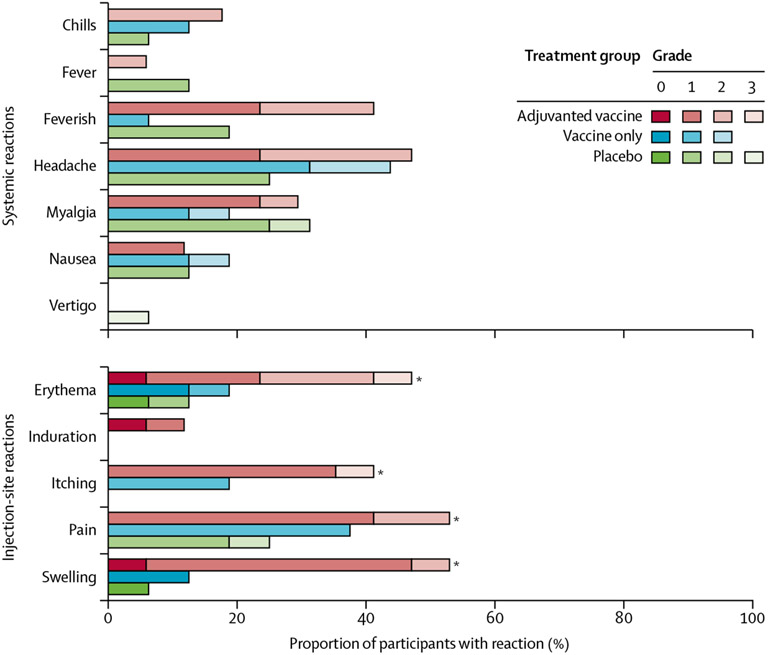
Figure 2: Adverse events after vaccine administration until 12-months of follow-up as a proportion of total study population.
Listed are all injection-site adverse events and the systemic adverse events that were deemed clinically relevant, reported by study participants or noted by study clinicians, during the vaccine follow-up period of 12 months. *p<0·1 comparing adjuvanted vaccine group with placebo group using one-sided Fisher’s exact test.