SUMMARY
In order to estimate influenza-associated excess mortality in southern Brazil, we applied Serfling regression models to monthly mortality data from 1980 to 2008 for pneumonia/influenza- and respiratory/circulatory-coded deaths for all ages and for those aged ⩾60 years. According to viral data, 73·5% of influenza viruses were detected between April and August in southern Brazil. There was no clear influenza season for northern Brazil. In southern Brazil, influenza-associated excess mortality was 1·4/100 000 for all ages and 9·2/100 000 person-years for persons aged ⩾60 years using underlying pneumonia/influenza-coded deaths and 10·0/100 000 for all ages and 86·6/100 000 person-years for persons aged ⩾60 years using underlying respiratory/circulatory-coded deaths. Influenza-associated excess mortality rates for southern Brazil are similar to those published for other countries. Our data support the need for continued influenza surveillance to guide vaccination campaigns to age groups most affected by this virus in Brazil.
Key words: Brazil, epidemiology, influenza
INTRODUCTION
Influenza is a respiratory infection annually associated with significant morbidity and mortality [1, 2] and quantifying this burden is critical to guide influenza vaccination policy [3]. Nevertheless, estimates of deaths attributable to influenza are difficult to estimate because influenza infections are often not laboratory confirmed or documented on hospital discharge forms and deaths certificates. Furthermore, a proportion of influenza-associated deaths occur because of pneumonia and bacterial co-infections [4, 5] or as a result of exacerbations of chronic disease such as congestive heart failure and chronic obstructive pulmonary disease, when the influenza virus is no longer detectable through routine clinical testing [6–8].
Statistical models based on time-series of mortality data have been used mainly in temperate and/or high-income countries to quantify the burden of influenza epidemics [9–12]. Data from tropical Singapore and subtropical Hong Kong estimated influenza-associated excess mortality rates in persons of all ages to be 2·9/100 000 and 4·1/100 000 person-years for underlying pneumonia/influenza deaths and 11·9/100 000 and 12·4/100 000 person-years for underlying circulatory/respiratory deaths [13, 14]. These estimates are comparable to estimates in temperate countries, such as the USA (2·4 and 9·0 influenza-associated deaths/100 000 person-years, for pneumonia/influenza and respiratory/circulatory outcomes, respectively) [15], despite differences in the timing of influenza seasons.
Nevertheless, it is not known if influenza-associated excess mortality rates in these high-income countries are comparable with rates from large, middle-income countries with multiple climate zones and spanning several latitudes. There is a paucity of data on the burden of seasonal influenza in tropical and subtropical areas of different income strata, especially in South America [16, 17]. Brazil is the largest country in South America, extends across more than 35° of latitude, and encompasses the equatorial Amazon rain forest in the north to subtropical and temperate zones in the south.
The Brazilian Government started influenza vaccination campaigns in 1999, targeting persons aged ⩾65 years because most of the influenza-associated mortality burden documented in temperate countries was concentrated in the elderly. In addition, vaccination campaigns targeted other groups vulnerable to complications of influenza infection (e.g. transplant patients, people living with HIV/AIDS, and patients with chronic pulmonary, cardiovascular, and renal diseases) and health professionals. In 2000, the Brazilian Government extended vaccine recommendations to persons aged ⩾60 years. During April–May, Brazil annually administers the Southern Hemisphere composition of the influenza vaccine free of charge throughout the country. The annual vaccine coverage in the elderly, the largest group targeted, ranged from 68% to 89% during 1999–2010 [18].
In this study, we use the Brazilian mortality statistics from 1980 to 2008 to estimate influenza-associated excess mortality during influenza epidemics for the southern region of Brazil.
METHODS
Influenza viral data
The Brazilian Ministry of Health initiated influenza surveillance in 2000. The national influenza surveillance system consisted of a network of sentinel sites (i.e. general care facilities and emergency departments) that collected an average of five clinical respiratory samples (nasopharyngeal aspirates or throat and nasal swabs) each week from patients with influenza-like illness. Samples were tested for influenza and other respiratory viruses at each state's public health laboratory through indirect immunofluorescence assay (IFA) for detection of respiratory viruses including influenza A and B. The influenza-positive samples and 10% of the negative IFA samples were further retested in one of the three Brazilian National Influenza Centres (NICs) by reverse transcription–polymerase chain reaction and samples which again tested positive for influenza were subtyped and sequenced. We analysed the monthly number of IFA-positive influenza samples from the states' public health laboratories database during 2000–2008 to describe the seasonal pattern of respiratory virus activity in southern and northern Brazil and to determine the time periods when excess pneumonia/influenza and respiratory/circulatory deaths could be attributed to influenza illness.
Geographical division
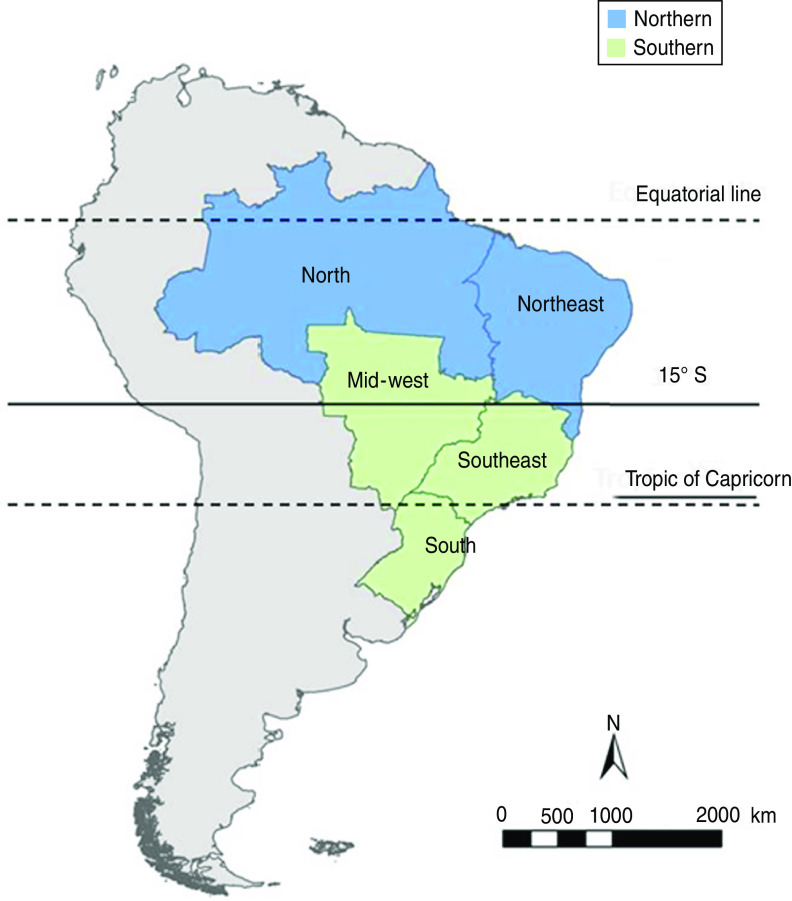
For the analyses, we divided Brazil into northern and southern regions which correspond to the 15o S latitude demarcating the different influenza seasonal patterns in tropical vs. subtropical/temperate Brazil [19] (Fig. 1). Southern Brazil included the mid-west, southeast and south; in 2010, this region had a population of 121 809 395 (11·5% aged ⩾60 years) and a mean population density of 39·1 persons/km2. Southern Brazil is where the country's major economics centres are located, with 91% of the population living in urban centres and a per capita GDP of US$12 768 [20]. The mean annual temperature in southern Brazil ranges between 14 °C and 24 °C and the annual rainfall ranges between 1250 mm and 2000 mm [21]. Northern Brazil included the north and northeast; in 2010, this region had a population of 68 946 404 inhabitants (9·5% aged ⩾60 years) and a mean population density of 12·7 persons/km2. Northern Brazil is less developed with 73% of the population living in urban centres and a per capita GDP of US$5058 [20]. The mean annual temperature in northern Brazil ranges between 20 °C and 28 °C and the rainfall can exceed 3000 mm during the first 6 months (January–June) of the year in parts of the Amazon [21].
Fig. 1.
[colour online]. Political division of Brazil and study stratification into northern and southern Brazil.
National mortality and population data
The National Vital Statistics Department of the Brazilian Ministry of Health provided national mortality data on pneumonia, influenza, respiratory, and cardiac-coded deaths [22]. Data were collected throughout the year using a standard form and database for the whole country. We categorized mortality data collected during 1980–1995 according to International Classification of Diseases, Ninth Revision (ICD-9) codes [23] and data collected during 1996–2008 according to Tenth Revision (ICD-10) codes [24]. We modelled two death categories according to the underlying cause of death: pneumonia/influenza mortality codes (i.e. ICD-9 codes 480–487 and ICD-10 codes J10–J18) and respiratory/circulatory mortality codes (i.e. ICD-9 codes 390–519 and ICD-10 codes I00–99 and J00–99). We used the census projections estimated in the first year of every decade for each subsequent year by the Brazilian Institute of Geography and Statistics to determine annual population denominators [20]. No visible change in annual pneumonia/influenza and respiratory/circulatory deaths was observed during the transition from ICD-9 to ICD-10, thus no adjustment was needed.
Statistical analyses
We applied Serfling regression models [25, 26] to monthly mortality data for persons of all ages and for those aged ⩾60 years, the main target group for influenza vaccination. The Serfling regression model is a linear regression model that includes terms for time trends and harmonic terms to model seasonal fluctuations in the number of deaths. It assumed a distinct influenza season and used only the monthly number of deaths that occurred outside the influenza season (defined by viral surveillance data) to estimate the seasonal pattern of deaths that were not associated with influenza. In these models:
where Yi represents the number of deaths in a particular month i, β0 represents the intercept, β1 represents a coefficient for the linear time trend, β2 represents a coefficient for the quadratic time trend, β3 and β4 represent the coefficients associated with the seasonal fluctuations in deaths, and ei represents the error term. Ninety-five percent confidence intervals (CIs) were generated for the prediction for the entire linear regression line which is larger than the estimated variance of the mean response. We defined the ‘epidemic months’ for each season as those months for which deaths exceeded the upper 95% confidence limit of deaths predicted by the model during the influenza season. The influenza-associated excess of deaths was determined by subtracting the observed deaths from the predicted deaths for the ‘epidemic months’. The annual rate of influenza-associated mortality/100 000 person-years was calculated by dividing the annual cumulative number of excess deaths during the influenza season by the sum of the annual mid-year population estimate for the period for all ages and in those aged ⩾60 years.
RESULTS
Timing of influenza activity in Brazil
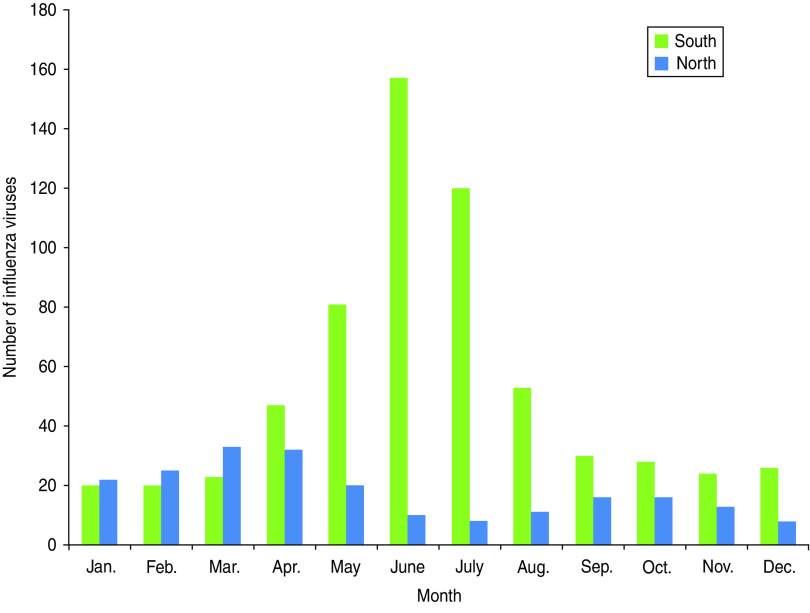
During 2000–2008, 841 influenza viruses were detected, 627 (75%) in southern Brazil and 214 (25%) in northern Brazil. Influenza was detected throughout the year in southern and northern Brazil. In southern Brazil, most positive samples (73·5%) were found during April–August (Fig. 2). Therefore, we considered the period of April–August as the epidemic season for southern Brazil in our model. In northern Brazil, however, there was insufficient viral data to clearly define the influenza season to the extent necessary for our Serfling models and we excluded this region from our analysis.
Fig. 2.
[colour online]. Number of influenza viruses identified through routine surveillance in southern and northern Brazil during 2000–2008.
Deaths by age group in southern Brazil
In southern Brazil, the annual median of pneumonia/influenza deaths in all ages was 27 419 (range 23 342–35 439) and in persons aged ⩾60 years it was 15 408 (range 6775–27 790). The annual median proportion of underlying pneumonia/influenza deaths in all decedents aged ⩾60 years was 55% (range 24% in 1980 to 78% in 2008). The annual median of underlying respiratory/circulatory deaths was 264 014 (range 208 023–300 707) and in persons aged ⩾60 years it was 185 464 (range 118 941–233 376). The annual median proportion of underlying respiratory/circulatory deaths in all decedents aged ⩾60 years was 70% (range 57% in 1980 to 78% in 2008).
Influenza-associated excess mortality rates in southern Brazil
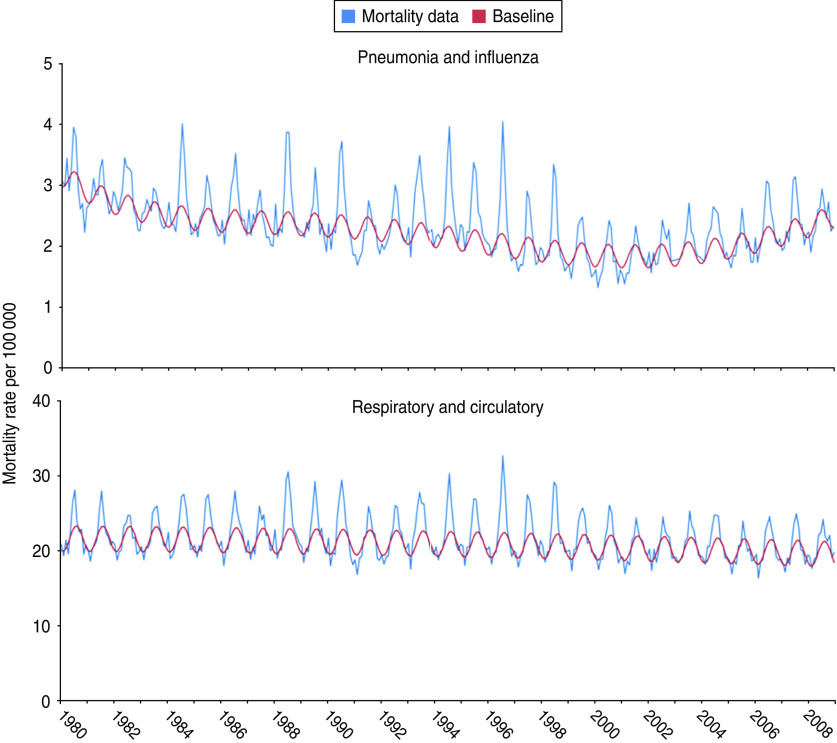
The models fitted the data well, especially for respiratory/circulatory data, R = 0·81 for all ages and R = 0·83 for persons aged ⩾60 years. For pneumonia/influenza data, R = 0·76 for all ages and R = 0·68 for persons aged ⩾60 years (Fig. 3). In southern Brazil, the annual median number of influenza-associated excess deaths estimated using underlying pneumonia/influenza data was 1035 [interquartile range (IQR) 445–2365] during the influenza season. These influenza-associated excess pneumonia/influenza deaths represented 1–12% of all pneumonia/influenza deaths coded each year. The annual rate of influenza-associated excess pneumonia/influenza mortality was 1·4 deaths/100 000 person-years [95% confidence interval (CI) 0·7–2·1 deaths/100 000 person-years] (Table 1). In persons aged ⩾60 years, the annual median number of influenza-associated excess pneumonia/influenza deaths was 498 (IQR 0–1495). The annual rate of influenza-associated excess pneumonia/influenza mortality for this age group was 10·0 deaths/100 000 person-years (95% CI 4·9–15·1 deaths/100 000 person-years) (Table 2).
Fig. 3.
[colour online]. Serfling models of excess mortality during the influenza season in southern Brazil for all ages during 1980–2008.
Table 1.
Total and excess deaths, and influenza-associated excess mortality (pneumonia/influenza and respiratory/circulatory) for all ages, 1980–2008 in southern Brazil
| Pneumonia and influenza | Respiratory and circulatory | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | Total number of deaths | Excess number of deaths | 95% CI | Excess death rate/100 000 | Excess deaths/ total deaths (%) | Total number of deaths | Excess number of deaths | 95% CI | Excess death rate/100 000 | Excess deaths/ total deaths (%) |
| 1980 | 28 661 | 1035 | 408–1661 | 1·3 | 3·6 | 209 424 | 6465 | 3349–9582 | 8·3 | 3·1 |
| 1981 | 27 419 | 347 | 40–654 | 0·4 | 1·3 | 209 393 | 5633 | 2508–8758 | 7·1 | 2·7 |
| 1982 | 27 305 | 1633 | 387–2878 | 2·0 | 6·0 | 208 023 | 0* | 0–0 | 0·0 | 0·0 |
| 1983 | 25 604 | 0* | 0–0 | 0·0 | 0·0 | 217 097 | 6000 | 1177–10 824 | 7·3 | 2·8 |
| 1984 | 27 481 | 2418 | 1449–3388 | 2·9 | 8·8 | 226 344 | 6955 | 3695–10 215 | 8·3 | 3·1 |
| 1985 | 25 710 | 466 | 135–797 | 0·5 | 1·8 | 229 221 | 7377 | 4031–10 724 | 8·7 | 3·2 |
| 1986 | 27 416 | 2012 | 674–3349 | 2·3 | 7·3 | 232 928 | 8354 | 3271–13 437 | 9·6 | 3·6 |
| 1987 | 25 267 | 0* | 0–0 | 0·0 | 0·0 | 231 983 | 2986 | 1249–4723 | 3·4 | 1·3 |
| 1988 | 29 127 | 2726 | 1689–3763 | 3·0 | 9·4 | 252 554 | 18 821 | 11 804–25 838 | 21·0 | 7·5 |
| 1989 | 26 362 | 678 | 326–1030 | 0·7 | 2·6 | 244 139 | 10 850 | 5 504–16 197 | 11·9 | 4·4 |
| 1990 | 28 265 | 2524 | 1453–3595 | 2·7 | 8·9 | 249 017 | 16 395 | 9149–23 641 | 17·7 | 6·6 |
| 1991 | 24 270 | 0* | 0–0 | 0·0 | 0·0 | 236 055 | 2898 | 1050–4745 | 3·1 | 1·2 |
| 1992 | 26 438 | 963 | 230–1696 | 1·0 | 3·6 | 246 826 | 5968 | 2248–9689 | 6·2 | 2·4 |
| 1993 | 29 902 | 3589 | 1724–5454 | 3·7 | 12·0 | 264 014 | 18 881 | 9400–28 362 | 19·4 | 7·2 |
| 1994 | 30 246 | 3353 | 2217–4489 | 3·4 | 11·1 | 264 868 | 15 281 | 9506–21 055 | 15·5 | 5·8 |
| 1995 | 29 463 | 2650 | 1495–3805 | 2·7 | 9·0 | 263 994 | 11 858 | 5986–17 729 | 11·9 | 4·5 |
| 1996 | 28 251 | 3473 | 2307–4640 | 3·4 | 12·3 | 274 042 | 20 409 | 14 484–26 333 | 20·2 | 7·4 |
| 1997 | 24 862 | 1440 | 645–2236 | 1·4 | 5·8 | 267 580 | 12 524 | 6502–18 546 | 12·2 | 4·7 |
| 1998 | 26 362 | 2365 | 1558–3171 | 2·3 | 9·0 | 276 137 | 17 090 | 10 961–23 219 | 16·4 | 6·2 |
| 1999 | 24 044 | 445 | 37–852 | 0·4 | 1·8 | 272 745 | 12 926 | 4674–21 179 | 12·2 | 4·7 |
| 2000 | 23 342 | 426 | 4–847 | 0·4 | 1·8 | 272 251 | 7365 | 3116–11 613 | 6·7 | 2·7 |
| 2001 | 24 000 | 0* | 0–0 | 0·0 | 0·0 | 271 459 | 2678 | 508–4848 | 2·4 | 1·0 |
| 2002 | 26 274 | 445 | 12–879 | 0·4 | 1·7 | 276 835 | 2928 | 728–5127 | 2·6 | 1·1 |
| 2003 | 27 586 | 724 | 285–1164 | 0·6 | 2·6 | 283 182 | 3886 | 1657–6116 | 3·4 | 1·4 |
| 2004 | 29 936 | 1661 | 325–2997 | 1·4 | 5·5 | 293 393 | 13 538 | 4514–22 562 | 11·8 | 4·6 |
| 2005 | 28 124 | 479 | 19–938 | 0·4 | 1·7 | 280 627 | 2905 | 576–5233 | 2·5 | 1·0 |
| 2006 | 33 469 | 1759 | 825–2694 | 1·5 | 5·3 | 293 384 | 9767 | 2655–16 880 | 8·1 | 3·3 |
| 2007 | 34 808 | 1623 | 672–2573 | 1·3 | 4·7 | 295 916 | 7832 | 3005–12 659 | 6·4 | 2·6 |
| 2008 | 35 439 | 0* | 0–0 | 0·0 | 0·0 | 300 707 | 6345 | 1526–11 163 | 5·2 | 2·1 |
CI, Confidence interval.
The 95% confidence intervals of modelled influenza excess deaths exceeded the observed number of deaths.
Table 2.
Total and excess deaths, and influenza-associated excess mortality (pneumonia/influenza and respiratory/circulatory) for persons aged ⩾60 years, 1980–2008 in southern Brazil
| Pneumonia and influenza | Respiratory and circulatory | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | Total number of deaths | Excess number of deaths | 95% CI | Excess death rate/100 000 | Excess deaths/total deaths (%) | Total number of deaths | Excess number of deaths | 95% CI | Excess death rate/100 000 | Excess deaths/total deaths (%) |
| 1980 | 6868 | 621 | 309–934 | 13·0 | 9·0 | 127 510 | 5457 | 3363–7551 | 114·3 | 4·3 |
| 1981 | 6794 | 0* | 0–0 | 0·0 | 0·0 | 128 768 | 5957 | 2744–9170 | 120·1 | 4·6 |
| 1982 | 6946 | 0* | 0–0 | 0·0 | 0·0 | 128 440 | 0* | 0–0 | 0·0 | 0·0 |
| 1983 | 8165 | 0* | 0–0 | 0·0 | 0·0 | 137 776 | 5384 | 1908–8860 | 99·5 | 3·9 |
| 1984 | 9049 | 0* | 0–0 | 0·0 | 0·0 | 143 473 | 4392 | 1989–6794 | 77·9 | 3·1 |
| 1985 | 9943 | 0* | 0–0 | 0·0 | 0·0 | 149 310 | 7202 | 3439–10 964 | 122·8 | 4·8 |
| 1986 | 10 897 | 334 | 139–529 | 5·5 | 3·1 | 151 774 | 5756 | 1848–9663 | 94·6 | 3·8 |
| 1987 | 10 860 | 0* | 0–0 | 0·0 | 0·0 | 154 104 | 3598 | 901–6295 | 57·0 | 2·3 |
| 1988 | 13 160 | 1308 | 684–1932 | 20·1 | 9·9 | 169 221 | 12 346 | 8158–16 534 | 189·3 | 7·3 |
| 1989 | 12 045 | 287 | 72–502 | 4·3 | 2·4 | 164 171 | 7995 | 3673–12 316 | 118·8 | 4·9 |
| 1990 | 14 257 | 1666 | 1003–2329 | 24·0 | 11·7 | 171 485 | 12 658 | 6719–18 596 | 182·5 | 7·4 |
| 1991 | 12 420 | 0* | 0–0 | 0·0 | 0·0 | 163 539 | 2247 | 711–3784 | 31·3 | 1·4 |
| 1992 | 13 689 | 490 | 14–966 | 6·5 | 3·6 | 171 752 | 3759 | 564–6954 | 50·1 | 2·2 |
| 1993 | 16 098 | 2267 | 1098–3437 | 30·8 | 14·1 | 185 464 | 16370 | 8521–24 219 | 222·4 | 8·8 |
| 1994 | 16 605 | 2213 | 1501–2924 | 29·7 | 13·3 | 186 128 | 13 203 | 8426–17 979 | 177·1 | 7·1 |
| 1995 | 16 605 | 2168 | 971–3364 | 28·7 | 13·1 | 187 154 | 12 602 | 6150–19 054 | 167·0 | 6·7 |
| 1996 | 16 699 | 2210 | 1413–3007 | 26·5 | 13·2 | 198 077 | 14 978 | 9629–20 326 | 179·5 | 7·6 |
| 1997 | 15 098 | 946 | 405–1488 | 11·2 | 6·3 | 194 815 | 8013 | 4379–11 647 | 94·7 | 4·1 |
| 1998 | 16 530 | 1642 | 1094–2190 | 19·2 | 9·9 | 202 001 | 13 802 | 8291–19 312 | 161·3 | 6·8 |
| 1999 | 15 408 | 588 | 33–1142 | 6·8 | 3·8 | 200 816 | 11 608 | 4199–19 016 | 134·2 | 5·8 |
| 2000 | 15 462 | 0* | 0–0 | 0·0 | 0·0 | 202 566 | 2353 | 249–4457 | 24·0 | 1·2 |
| 2001 | 16 533 | 0* | 0–0 | 0·0 | 0·0 | 203 040 | 0* | 0–0 | 0·0 | 0·0 |
| 2002 | 18 702 | 0* | 0–0 | 0·0 | 0·0 | 209 615 | 0* | 0–0 | 0·0 | 0·0 |
| 2003 | 20 061 | 498 | 174–823 | 4·9 | 2·5 | 215 111 | 3076 | 897–5254 | 30·3 | 1·4 |
| 2004 | 22 287 | 1495 | 512–2477 | 14·5 | 6·7 | 224 557 | 12 699 | 3904–21 494 | 123·6 | 5·7 |
| 2005 | 21 202 | 506 | 170–843 | 4·8 | 2·4 | 215 505 | 3260 | 1001–5519 | 31·0 | 1·5 |
| 2006 | 25 665 | 2761 | 1396–4125 | 25·9 | 10·8 | 226 513 | 14 172 | 5014–23 330 | 133·0 | 6·3 |
| 2007 | 27 211 | 1216 | 401–2031 | 9·7 | 4·5 | 229 454 | 0* | 0–0 | 0·0 | 0·0 |
| 2008 | 27 790 | 0* | 0–0 | 0·0 | 0·0 | 233 376 | 0* | 0–0 | 0·0 | 0·0 |
CI, Confidence interval.
The 95% confidence intervals of modelled influenza excess deaths exceeded the observed number of deaths.
The annual median number of influenza-associated excess deaths in all ages estimated using underlying respiratory/circulatory data was 7377 (IQR 5633–12 926) during the influenza season. These influenza-associated excess deaths represented 1–7% of all respiratory/circulatory deaths coded each year. The annual rate of influenza-associated excess respiratory/circulatory mortality was 9·2 deaths/100 000 person-years (95% CI 4·5–13·9 deaths/100 000 person-years) (Table 1). In persons aged ⩾60 years the annual median number of influenza-associated excess respiratory/circulatory deaths was 5761 (IQR 3088–12 345). The annual rate of influenza-associated excess respiratory/circulatory mortality for this age group was 86·6 deaths/100 000 person-years (95% CI 41·6–131·7 deaths/100 000 person-years) (Table 2).
We conducted additional sensitivity analysis for our models, considering May–August as the epidemic period and using a 99% CI to define the ‘epidemic months’ and found similar results with no significant statistical difference from the results presented (data not shown).
DISCUSSION
We present estimates of influenza-associated excess mortality for two categories of underlying cause of death codes for southern Brazil. The estimates of respiratory/circulatory deaths are more likely to provide an estimate of the full range of influenza-associated deaths than estimates made with pneumonia/influenza data because the former category includes deaths caused by exacerbations of chronic cardiac and respiratory diseases which can be secondary to influenza infection [15]. Our estimates of influenza-associated excess mortality for all ages and the elderly in southern Brazil are similar to rates in tropical Singapore, subtropical Hong Kong and even to temperate countries like the USA [13–15] (Table 3). South Africa, however, a country at a similar latitude (22o S to 35o S) and income level [27] to southern Brazil had a higher influenza-associated excess pneumonia/influenza mortality during 1998–2005 (i.e. 42/100 000 in persons aged ⩾65 years) [28] than southern Brazil (i.e. 10·0 deaths/100 000 in persons aged ⩾60 years) and other countries as well. Difference in age group composition, lower influenza vaccine coverage, limited access to healthcare, high prevalence of underlying illnesses and malnutrition, and crowded living conditions in parts of South Africa may be attributed to their higher influenza mortality rate [28, 29]. One study performed in the city of São Paulo, the main urban centre located in southern Brazil, from 1993–2002 using a Serfling regression model and assuming influenza seasonal periods where observed deaths rates exceeded the epidemic threshold for at least two consecutive weeks, found similar rates of influenza-associated excess pneumonia/influenza mortality for those aged ⩾65 years, with yearly variation from 0·0 to 24·1/100 000 inhabitants [30].
Table 3.
Influenza-associated excess mortality rates per 100 000 person-years in southern Brazil, Singapore, Hong Kong, USA and South Africa
| Country | Period of study | Statistical method | Underlying pneumonia and influenza deaths (/100 000 person-years) | Underlying respiratory and circulatory deaths (/100 000 person-years) |
|---|---|---|---|---|
| Southern Brazil | 1980–2008 | Serfling regression model using monthly number of deaths | All ages: 1·4 ⩾60 yr: 10·0 | All ages: 9·2 ⩾60 yr: 86·6 |
| Singapore [13] | 1996–2003 | Negative binominal regression model using monthly number of deaths and monthly proportion of positive influenza test results | All ages: 2·9 ⩾65 yr: 46·9 | All ages: 11·9 ⩾65 yr: 155·4 |
| Hong Kong [14] | 1996–1999 | Poisson regression model using weekly number of deaths and weekly proportion of positive influenza test results | All ages: 4·1 ⩾65 yr: 39·3 | All ages: 12·4 ⩾65 yr: 102·0 |
| USA [15] | 1976–2007 | Poisson regression model using weekly number of deaths and weekly proportion of positive influenza test results | All ages: 2·4 ⩾65 yr: 17·0 | All ages: 9·0 ⩾65 yr: 66·1 |
| South Africa [28] | 1998–2005 | Serfling regression model using monthly number of deaths | ⩾65 yr: 42·0 |
There was year-to-year variability in influenza-associated mortality estimates in southern Brazil. Seasons with highest estimates of excess deaths are characterized by a dominance of influenza A(H3N2) influenza viral subtype [13–15]. We worked with IFA data from the states' public health laboratories, thus we do not have viral data on influenza dominant strains for each season. Nevertheless, according to data published from two Brazilian NICs that studied 29 isolates from Belém, northern Brazil and 38 isolates from São Paulo, southern Brazil, from 1999 to 2007, the years with highest influenza-associated mortality in southern Brazil correspond to the years with the detection of new strains of influenza A(H3N2) in São Paulo (A/Fujian/411/2002 in 2004, A/California/7/2004 and A/Wisconsin/67/2005 in 2006) [31].
We used Serfling regression models which are useful when there is no robust viral data available. This model is simpler when compared to other regression models because it assumes that influenza mortality is seasonal and the impact of respiratory syncytial virus (another respiratory virus capable of causing severe disease in infants and the elderly) is accounted for in the baseline. Nevertheless, studies from the Northern and Southern Hemispheres have demonstrated that estimates of influenza-associated mortality are similar between the Serfling regression models and other methods [32, 33]. This method is often applied in temperate countries with distinct influenza seasons. However, such models may not be the best for tropical and subtropical areas where influenza may circulate throughout much of the year [16, 17]. Indeed, we found that influenza viruses circulate throughout the year in southern Brazil, and thus our baseline periods probably included periods when influenza-associated deaths occurred. This bias would lead to underestimates of influenza-associated mortality rates. Nevertheless, viral surveillance data paralleled mortality data. For example, during 2000–2008, pneumonia/influenza and respiratory/circulatory mortality peaked once during June–July in southern Brazil. Moreover, Alonso et al. in a study that addressed influenza seasonality in Brazil using mortality data from 1979 to 2001, observed a peak in pneumonia/influenza mortality in the same period in southern Brazil [19]. Distinct influenza seasonality was more difficult to discern in northern Brazil given the available viral data, which may be due in part a more complex, year-round influenza activity near the equator. Alonso et al. did not find evidence of variations in the amplitude of the seasonal cycles in the northern equatorial zones, but found only a peak of pneumonia/influenza deaths during April–May in the northern states of Brazil [19]. For these reasons, we did not apply our models, which rely on defined influenza seasonality, to the northern region of Brazil.
The Brazilian Mortality System has limitations. Although the coverage of the system has steadily improved, the proportion of deaths reported to the Brazilian Mortality System reached about 100% of the mortality estimated by the Brazilian Institute of Geography and Statistics in southern Brazil by the early 1990s. Before that period, there was underreporting of deaths to the system and a higher proportion of ill-defined coded diseases, especially in the elderly [34, 35]. This may explain the poorer adjustment of the model's baseline in the earliest years and the underestimation of influenza-associated mortality in the 1980s and early 1990s.
In conclusion, the mortality burden of influenza in southern Brazil appears comparable with that found in other countries with different climates and incomes. These data need to be confirmed in the future as more robust viral data become available in Brazil. Future investments in influenza surveillance are needed, especially in northern tropical Brazil, to better understand the seasonality and associated burden of influenza in this region. Vaccine effectiveness studies are necessary as Brazil will face an even greater influenza-associated burden as its population grows older. Increasing vaccination coverage may be a strategy to further decrease the burden of influenza-associated mortality, especially in those at highest risk of death as a result of infection.
ACKNOWLEDGEMENTS
The authors thank Nancy Cox, Ann Moen, Joseph Bresee, Otavio Olivia, Tomas Rodriguez and Marcia Lopes de Carvalho for their support and cooperation which made this manuscript possible, and Patricia Marques Ferreira for providing assistance with the figures.
The opinions expressed by authors contributing to this work do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Simonsen L, et al. The impact of influenza epidemics on mortality: introducing a severity index. American Journal of Public Health 1997; 87: 1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonsen L, et al. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. Journal of Infectious Diseases 1998; 178: 53–60. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Adoption of global agenda on influenza. Weekly Epidemiological Record 2002; 77: 191–195.12073536 [Google Scholar]
- 4.Bisno AL, et al. Pneumonia and Hong Kong influenza: a prospective study of the 1968–69 epidemic. American Journal of the Medical Sciences 1971; 116: 51–53. [DOI] [PubMed] [Google Scholar]
- 5.Gupta RK, George R, Nguyen-Van-Tam JS. Bacterial pneumonia and pandemic influenza planning. Emerging Infectious Diseases 2008; 14: 1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichol KL, Baken L, Nelson A. Relation between influenza vaccination and outpatient visits, hospitalization, and mortality in elderly persons with chronic lung disease. Annals of Internal Medicine 1999; 130: 397–403. [DOI] [PubMed] [Google Scholar]
- 7.Upshur REG, Knight K, Goel V. Time-series analysis of the relation between influenza virus and hospital admissions of the elderly in Ontario, Canada, for pneumonia, chronic lung disease, and congestive heart failure. American Journal of Epidemiology 1999; 149: 85–92. [DOI] [PubMed] [Google Scholar]
- 8.Griffin MR, et al. Winter viruses: influenza and respiratory syncytial virus related morbidity in chronic lung disease. Archives of Internal Medicine 2002; 162: 1229–1236. [DOI] [PubMed] [Google Scholar]
- 9.Alling DW, Blackwelder WC, Stuart-Harris CH. A study of excess mortality during influenza epidemics in the United States, 1968–1976. American Journal of Epidemiology 1981; 113: 30–43. [DOI] [PubMed] [Google Scholar]
- 10.Lui KJ, Kendal AP. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. American Journal of Public Health 1987; 77: 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichert T, et al. Influenza and the winter increase in mortality in the United States, 1959–1999. American Journal of Epidemiology 2004; 160: 492–502. [DOI] [PubMed] [Google Scholar]
- 12.Thompson WW, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. Journal of the American Medical Association 2003; 289: 179–86. [DOI] [PubMed] [Google Scholar]
- 13.Chow A, et al. Influenza-associated deaths in tropical Singapore. Emerging Infectious Diseases 2006; 12: 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong CM, et al. Influenza-associated mortality in Hong Kong. Clinical Infectious Diseases 2004; 39: 1611–1617. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Estimates of deaths associated with seasonal influenza – United States, 1976–2007. Morbidity and Mortality Weekly Report 2010; 59: 1057–1062. [PubMed] [Google Scholar]
- 16.Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Medicine 2006; 3: e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moura FEA. Influenza in the tropics. Current Opinion in Infectious Diseases 2010; 23: 415–420. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho MTB, et al. The National Immunization Program in the 20 years of Unified Health System in Brazil. In: Health Brazil 2008: 20 years of Unified Health System (SUS) in Brazil. Brasilia: Ministry of Health, 2009, pp. 63–83. [Google Scholar]
- 19.Alonso WJ, et al. Seasonality of influenza in Brazil: a traveling wave from the Amazon to the subtropics. American Journal of Epidemiology 2007; 165: 1434–1442. [DOI] [PubMed] [Google Scholar]
- 20.Brazilian Institute of Geography and Statistics. Censo demografico 1980, 1990, 2000. (http://www.ibge.gov.br). Accessed 25 November 2010.
- 21.National Institute of Meteorology. (http://www.inmet.gov.br). Accessed 21 May 2011.
- 22.Brazilian Ministry of Health. Vital Statistics Brazil, DATASUS (http://www.datasus.gov.br). Accessed 1 December 2010.
- 23.World Health Organization. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death, Based on Recommendations of the Ninth Revision Conference, 1975. Geneva: World Health Organization; 1977. [Google Scholar]
- 24.World Health Organization. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death, Based on Recommendations of the Tenth Revision Conference, 1992. Geneva: World Health Organization; 1992. [Google Scholar]
- 25.Serfling RE. Methods for current statistical analysis of excess pneumonia-influenza deaths. Public Health Reports 1963; 78: 494–506. [PMC free article] [PubMed] [Google Scholar]
- 26.Serfling RE, Sherman IL, Houseworth WJ. Excess pneumonia-influenza mortality by age and sex in three major influenza A2 epidemics, United States, 1957–58, 1960 and 1963. American Journal of Epidemiology 1967; 86: 433–441. [DOI] [PubMed] [Google Scholar]
- 27.The World Bank. (http://data.worldbank.org/about/country-classifications/country-and-lending-groups#Upper_middle_income). Accessed 19 January 2011.
- 28.Cohen C, et al. Elevated influenza-related excess mortality in South African elderly individuals, 1998–2005. Clinical Infectious Diseases 2010; 51: 1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Macroepidemiology of Influenza Vaccination (MIV) Study Group. The macroepidemiology of influenza vaccination in 56 countries, 1997–2003. Vaccine 2005; 23: 5133–5143. [DOI] [PubMed] [Google Scholar]
- 30.Antunes JL, et al. Effectiveness of influenza vaccination and its impact on health inequalities. International Journal of Epidemiology 2007; 36: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 31.de Mello WA, et al. The dilemma of influenza vaccine recommendations when applied to the tropics: the Brazilian case examined under alternative scenarios. PLoS One 2009; 4: e5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson WW, et al. Estimates of US influenza-associated deaths made using four different methods. Influenza and Other Respiratory Viruses 2009; 3: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newall AT, Viboud C, Wood JG. Influenza-attributable mortality in Australians aged more than 50 years: a comparison of different modelling approaches. Epidemiology and Infection 2010; 138: 836–842. [DOI] [PubMed] [Google Scholar]
- 34.Inter-Agency Health Information Network (RIPSA). Basic Indicators for Health in Brazil: Concepts and Applications, 2nd edn. Brasilia: Pan-American Health Organization, 2008, pp. 292–293. [Google Scholar]
- 35.Mello-Jorge MHP, Gotlieb SLD, Laurenti R. The national mortality information system: problems and proposals for solving them. I – Deaths by natural causes. Revista Brasileira de Epidemiologia 2002; 5: 197–211. [Google Scholar]