SUMMARY
Sentinel species are increasingly used by disease managers to detect and monitor the prevalence of zoonotic diseases in wildlife populations. Characterizing home-range movements of sentinel hosts is thus important for developing improved disease surveillance methods, especially in systems where multiple host species co-exist. We studied ranging activity of major hosts of bovine tuberculosis (TB) in an upland habitat of New Zealand: we compared home-range coverage by ferrets (Mustela furo), wild deer (Cervus elaphus), feral pigs (Sus scrofa), brushtail possums (Trichosurus vulpecula) and free-ranging farmed cattle (Bos taurus). We also report in detail the proportional utilization of a seasonal (4-monthly) range area for the latter four species. Possums covered the smallest home range (<30 ha), ferrets covered ∼100 ha, pigs ∼4 km2, deer and cattle both >30 km2. For any given weekly period, cattle, deer and pigs were shown to utilize 37–45% of their estimated 4-month range, while possums utilized 62% during any weekly period and 85% during any monthly period of their estimated 4-month range. We suggest that present means for estimating TB detection kernels, based on long-term range size estimates for possums and sentinel species, probably overstate the true local surveillance coverage per individual.
Key words: Disease sentinels, disease surveillance, home-range analysis, New Zealand, probability of eradication, tuberculosis, wildlife
INTRODUCTION
Bovine tuberculosis (TB) is caused by an obligate parasite bacillus, Mycobacterium bovis, a zoonotic pathogen that has a particularly wide mammalian host range [1, 2]. In New Zealand, that host range includes not only farmed cattle and deer, but also a large number of introduced wild mammals, most notably brushtail possums (Trichosurus vulpecula), pigs (Sus scrofa), ferrets (Mustela furo) and wild red deer (Cervus elaphus) [3]. Possums are the only wild species considered to be true TB maintenance hosts in New Zealand [4, 5], since the densities at which pigs, ferrets and deer occur in the wild are generally below the level at which they can independently maintain the M. bovis infection cycle [5–7]. Consistent with that paradigm, a national programme combining TB testing of livestock (with slaughter of test-positive animals) and intensive lethal control of possum populations has resulted in a >95% reduction in TB levels in New Zealand's farmed livestock since 1995 [8].
The decline in TB levels in both livestock and wildlife from large areas of New Zealand has prompted a progressive shift in the national disease management strategy, away from simply applying lethal control of possums to break the disease maintenance cycle and towards a mix of continued control coupled with TB surveillance [9]. However, because infected possum populations have already been reduced to very low levels, and because the animals themselves have small home ranges (typically <30 ha [10]), direct surveillance of possum populations for the presence of TB using classical sampling theory can be imprecise [11, 12] and is becoming increasingly expensive. Surveillance for TB is therefore becoming increasingly focused not only on possums themselves, but additionally on the use of other wildlife species as ‘sentinels’ for the ongoing presence of M. bovis in the environment. A key advantage of using sentinel species is that they can be equally, or more, susceptible to becoming infected by an encounter with a tuberculous possum as can possums themselves, but range more widely (thus effectively ‘searching’ for the presence of TB across a much wider area of the landscape). An understanding of the home-range size of, and temporal patterns of home-range utilization by, TB sentinels – relative to range-use patterns of the primary host (possums) – is thus important in efforts to manage the disease in the environment by means of improved sentinel-based surveillance. Pigs are recognized as being particularly good sentinels for these reasons [11], and both pigs and ferrets have been used to indicate the presence of TB in New Zealand [13], while in North America, wild coyotes (Canis latrans) have been reported for use as sentinels for the presence of TB in wild cervid populations [14–16].
In the New Zealand case, where the main reservoir species (the possum) is being controlled and the incidence of TB is declining, future surveys involving sentinel species will aim to determine more the presence or absence of disease, rather than its prevalence. Interpreting the outcome of sentinel surveys where TB is found is comparatively straightforward – it would usually indicate that further possum control and disease surveillance are required. Interpreting the absence of TB in surveyed sentinels is more complex, both in terms of (a) assessing the probability that there truly is no TB persisting in the area surveyed, and also (b) delineating the area that has been effectively ‘searched’ by the sentinel. New concepts based on detection kernels have been developed to account for this, whereby the ‘search effort’ for TB of the sentinel is modelled as a function of its home-range size and the force of infection experienced by that species when a single tuberculous possum is present within the sentinel's home range [17]. Within the resulting ‘Proof of TB Freedom’ framework [17, 18], the relative utility of sentinels in providing spatially explicit inference about the likely TB status of the possum population is thus strongly linked to the sentinels’ home-range size. In this paper we therefore present the first comparison, under similar environmental conditions, of ranging behaviour of possums themselves relative to that of the four species most likely to be used as possum TB sentinels (wild red deer, wild ferrets, feral pigs, free-ranging farmed cattle). We focus predominantly on short-term (weekly and monthly) ranging behaviour partly for technical reasons, but also, and more importantly, because that is the range size that appears most relevant in the detection of TB in possums (see Discussion). Our study was not designed in advance but, rather, makes use of movement data collected as part of a series of separate but often interconnected studies. As a consequence, some of our analyses are constrained to, for example, particular subsets of the hosts studied, or to particular seasons.
METHODS
Study area
The study area was centred within the northern South Island high country (NSIHC) of New Zealand (42° S, 173° E; see map in the Supplementary Material for location, study sites and trapping/monitoring areas). The NSIHC area covers some 5000 km2, within which are large farming stations, including Muzzle and Molesworth stations on which this study was conducted. Beef cattle are grazed at a low density (<5/km2) throughout these stations, nominally within 5000–10 000 ha regions bounded by natural features (ravines, major rivers, high ridgelines) but otherwise free to range. We used several study sites (see below and map in the Supplementary Material) within the NSIHC area, but all sites comprised a similar mix of largely unforested, semi-arid mountainous habitats (elevation range 550–2100 m) in which the vegetation is dominated by low-lying shrubs and grasses, interspersed with rose briar, matagouri and other scrubby plants (see [19] for full description). The study sites hold a low-density unmanaged population of possums (typically <1/ha [20]), low to moderate densities of ferrets (2–4/km2 [21]) and feral pigs (1–2/km2 [20]), and a low density, widely distributed population of wild red deer (<1/km2 [22]). Investigations in the NSIHC area, since 2000, have recorded TB in all five species, with the cattle herds being among the most heavily infected livestock herds remaining in New Zealand, and with very high levels of infection recorded in pigs [7, 20, 22].
Radio tracking
For collar-fitting, possums were captured using soft-catch leg-hold traps [19], while ferrets were captured using cantilever-type cage traps [20]. Deer were captured using tranquillizer darts fired from a helicopter. Pigs were bred specifically for the trial from pen-maintained wild pigs sourced from the area, as described previously [7]. Cattle steers were fitted with collars during an annual muster for TB testing. For all species, fix-point data were acquired and utilized within 3 weeks of the first fitting of the collars. All procedures were approved by the Landcare Research Animal Ethics Committee.
Two types of collars were used to track animals: either a commercially available GPS package (Sirtrack NZ Ltd, New Zealand) or a homebuilt Landcare Research version. Both collar types could be tracked externally by VHF telemetry (if required) but primarily provided automated GPS data on the date, time, latitude, longitude, and horizontal dilution of precision (HDOP; a measure of accuracy) for each fix, with up to 20 fix points per day recorded. An initial screening to identify low-accuracy fixes focused on those locations with large HDOP values (⩾10 for Sirtrack collars, ⩾30 for homebuilt collars) [23]. Because this method of screening for inaccurate fixes is also prone to discarding accurate ones [24, 25], we visually assessed fixes with high HDOP in relation to the previous and subsequent 10 locations for that animal. If the fix with high HDOP was located within the vicinity of the other locations, it was considered to be biologically reasonable and retained for analysis, otherwise it was discarded.
For ferrets, and for one set of possums (n = 29), tracking data were obtained via VHF telemetry only, by attempting to locate each animal during the day at 6- to 8-week intervals between March 2005 and March 2006, either by an observer on foot or from a helicopter. For cattle, deer, pigs, and a second set of possums (n = 27), more detailed location data were obtained by GPS to obtain multiple fixes per day for each animal. The GPS data were collected over a 6-month period for possums (between September 2009 and February 2010); and over 14-month periods for wild red deer, free-ranging cattle and feral pigs (between May 2009 and July 2010 for feral pigs, September 2009 and October 2010 for cattle, and between January 2010 and February 2011 for deer).
Spatial analyses
Location data were used to estimate home ranges using either a 100% minimum convex polygon (MCP) or a 95% isopleth fixed-kernel density estimator (KDE). Both analyses were conducted in Home Range Tools for ArcGIS [26], using least-squares cross-validation to estimate the smoothing factor h for KDEs [27, 28]. All spatial analyses were performed using ArcGIS v. 9.3 [29].
In addition to estimating overall home ranges for all five species, we also investigated the short-term temporal patterns of home-range utilization by individuals of four species (cattle, deer, pigs, possums) tracked by GPS, by focusing on animals for which we had four consecutive months of observations with at least one fix per day and a minimum of 40 fixes per month [30, 31]. We calculated 95% KDE-based home-range estimates over the total 4-month period for each individual; then, we further assessed home-range size estimates for each animal over shorter periods by progressively truncating the length of its monitoring period from 4 months' worth of data to the first 3 months of monitoring, then 2 months, 1 month, 3 weeks, 2 weeks and finally to 1 week (see Results section, Fig. 2). In all cases, we selected these periods (4 months to 1 week) from the same broad seasonal time-span [i.e. spring (September) until mid-summer (February)] to minimize effects of inter-species variability due to seasonality. We then used these data to assess the rapidity by which each species increases its utilization of the total recorded 4-month range.
Fig. 2.
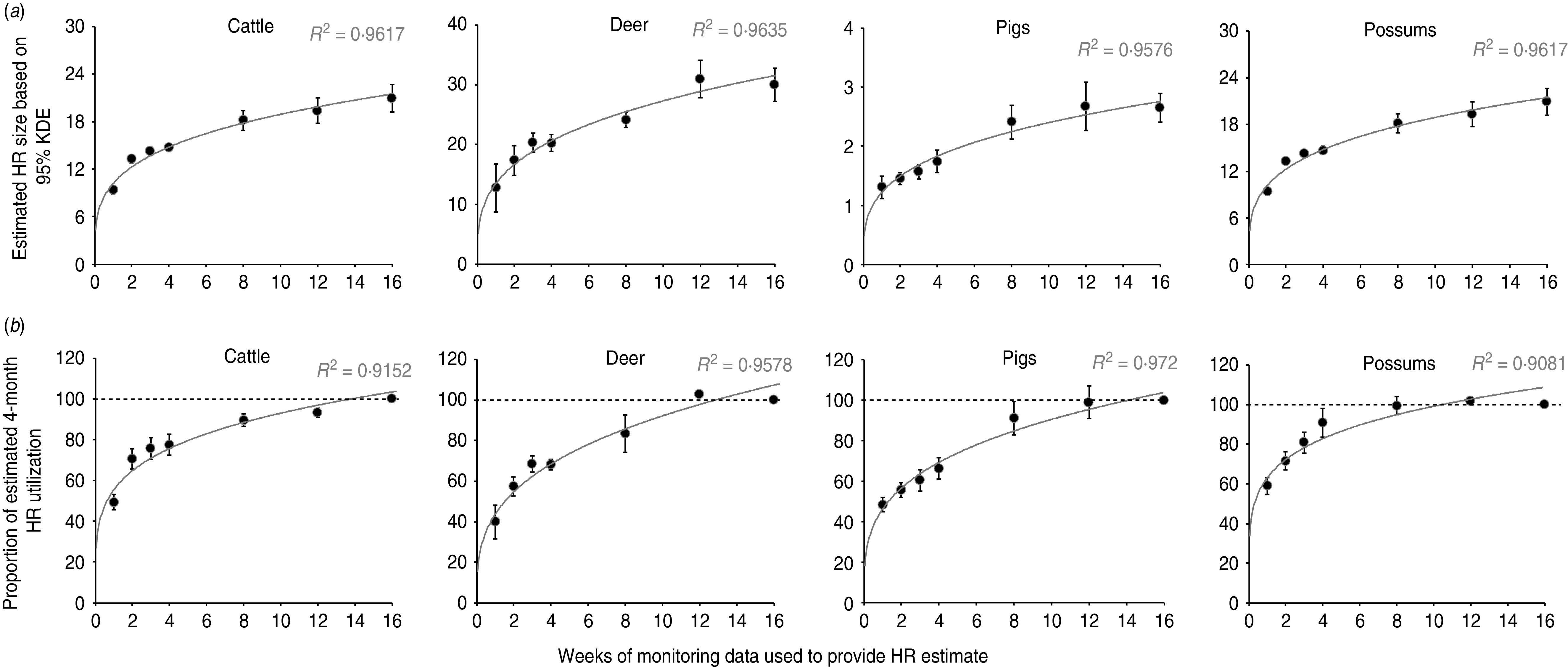
Patterns of home-range expansion (in both average size and proportional utilization) over a 4-month monitoring period, for four intensively (GPS) monitored mammalian species (n = 50) sharing similar semi-arid scrub/grassland habitat within the same geographical area. (a) The top row represents average home-range size for each species (±s.e.m.), based on increasing periods of animal monitoring (note that all areas are depicted in km2 except for possums, which are in hectares). (b) The bottom row represents average proportional utilization of the estimated 4-month home range (±s.e.m.) by each species over increasing periods of animal monitoring (the dashed line represents 100% utilization). Curved lines describe exponential relationships between dependent and independent variables, with R2 values referring to the coefficient of determination of the fitted line. HR, Home range; KDE, kernel density estimator.
In a subtly different analysis, we aimed to assess the proportion of the estimated 4-month home range that each individual animal might utilize during any given week or month within that 4-month monitoring period, rather than just the first week or first 4 weeks of its data; this was to provide an indication of average percentage utilization of total range. To achieve this, shorter time blocks of 1 week (16 blocks) and 4 weeks (four blocks) were identified within the 4-month period, and used to estimate area size for any given 1-week or 4-week block; average weekly and monthly percentage utilization within the 4 months was then calculated as the average for each block (see Results section, Fig. 3).
Fig. 3.
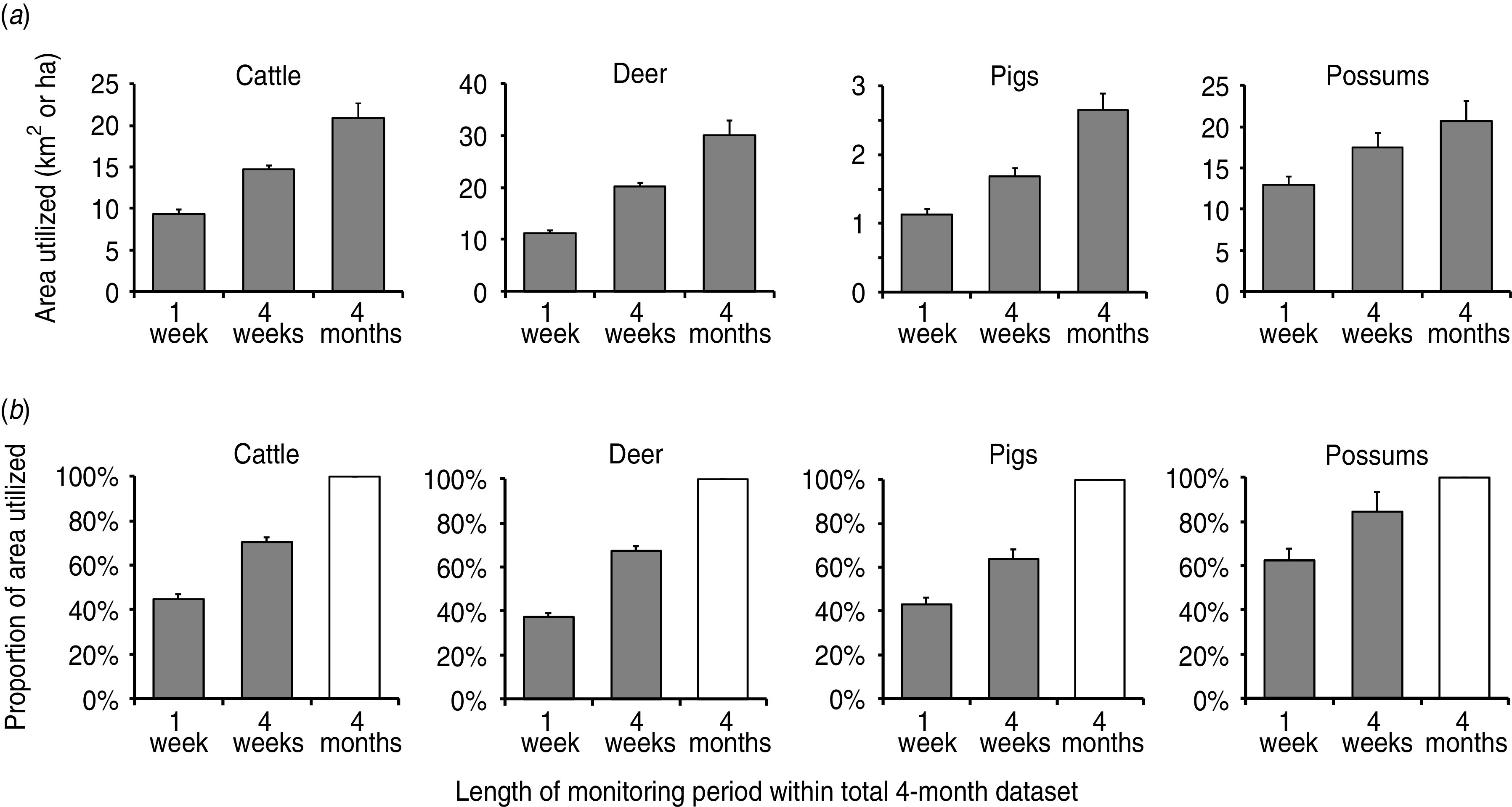
Short-term temporal (weekly, monthly, and four-monthly) patterns of area utilization (in both absolute terms and as a proportion of the 4-month home range) for four intensively monitored (GPS) mammalian species (n = 50) sharing similar semi-arid scrub/grassland habitat within the same geographical area. (a) The top row represents average areas (±s.e.m.) utilized by each species over any given 1-week or 4-week block during the 4-month period of monitoring (note that all areas are depicted in km2 except for possums, which are in hectares). (b) The bottom row represents the average proportional utilization of the estimated 4-month home range (±s.e.m.) by each species over any given 1-week or 4-week block during the 4-month monitoring period.
RESULTS
Home-range sizes
We obtained tracking data for 117 individual mammals in this study (Table 1). We obtained at least 20 locational fixes via GPS from 23 cattle, 13 deer, 13 pigs and 27 possums and used these data to provide KDE-based home-range estimates. We collected smaller sets of locational fixes via VHF telemetry for a further 29 possums and 12 ferrets, and used these data to provide MCP-based home-range estimates (Table 1, Fig. 1).
Table 1.
Summary of average estimated home-range sizes, based on total monitoring period, for five mammalian species
| Species, number (sex) | Average no. fixpoints per animal(min–max) usedto calculate range | Average total home range over monitoring period(95% confidence interval) | ||
|---|---|---|---|---|
| All animals | Males | Females | ||
| Cattle, n = 23 (all M) | 801 (75–1593) | 35·8 km2 (29·4–42·2) | 35·8 km2 (29·4–42·2) | — |
| Deer, n = 13 (5M, 8F) | 474 (37–1529) | 37·8 km2 (31·8–43·9) | 39·7 km2 (26·1–43·9) | 34·7 km2 (26·4–43·0) |
| Pigs, n = 13 (6M, 7F) | 337 (42–704) | 3·77 km2 (2·69–4·48) | 4·27 km2 (2·36–6·18) | 3·17 km2 (1·80–4·55) |
| Possums I, n = 27 (15M, 12F) | 397 (61–544) | 22·3 ha (16·7–28·0) | 27·8 ha (18·8–36·8) | 15·5 ha (10·2–20·8) |
| Possums II, n = 29 (14M, 15F) | 6 (4–7) | 5·14 ha (2·85–7·42) | 7·13 ha (2·93–11·33) | 3·28 ha (1·12–5·43) |
| Ferrets, n = 12 (5M, 7F) | 5 (4–6) | 104·4 ha (46·4–162·4) | 178·2 ha (63·4–294·3) | 51·6 ha (12·6–90·6) |
Data represent mean home-range estimates (95% confidence intervals) for each species. Analyses are based on 95% isopleth kernel density estimator calculations for cattle, deer, pigs and possums I, and on 100% minimum complex polygons calculations for possums II and ferrets.
Possums I monitored by GPS (which provided 24-hourly fix points per day); Possums II monitored by VHF telemetry (which gave only daytime, i.e. denning site, fix points).
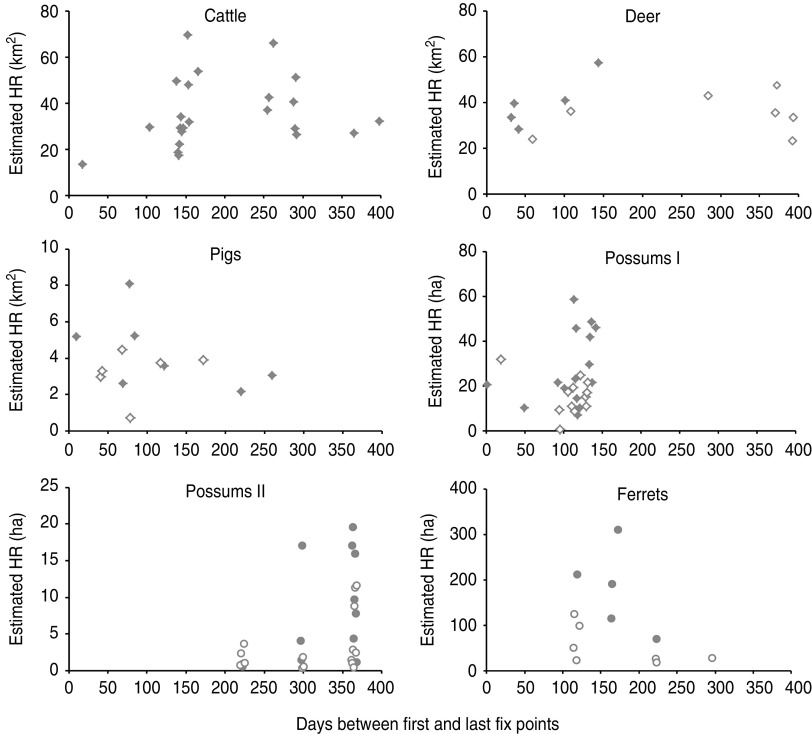
Fig. 1.
Estimates of individual home-range (HR) sizes for 117 individual animals of five mammalian species sharing the same geographical area, based on the interval between first and last fix-point total for each individual. Data are either 95% isopleth kernel density estimates (represented by diamond symbols for cattle, deer, pigs and one set of possums that were monitored using GPS), or 100% minimum convex polygon areas (represented by circular symbols for ferrets and a second set of possums that were monitored by VHF telemetry). Open symbols represent females, closed symbols represent males.
There was wide variation within and between species in the estimates of home-range size, with (for each species) the estimated area covered by males tending to be larger than females (Fig. 1). Cattle and deer had very large home ranges, with some individuals covering over 60 km2. Possums had the smallest home ranges, an average of 22·3 ha estimated from GPS data (based on 95% KDE) and 5·14 ha estimated from VHF telemetry fixes (100% MCP). The differences between these two estimates reflects the fact that GPS provided multiple fixes for 24 h per day, whereas VHF telemetry data could only be obtained during daylight hours, when strictly nocturnal possums were in daytime dens.
Temporal patterns in home-range utilization
We had a minimum of 4 months' worth of contiguous fix data for 21 cattle, four deer, five pigs and 20 possums. All four species showed a similar pattern of covering progressively larger areas over time, but with most of the increase occurring during the first 2 months of the monitoring period (Fig. 2). For possums, range expansion appeared to cease at 1–2 months, whereas pigs showed progressive range expansion from 1 week up to 4 months of monitoring; deer and cattle showed an intermediate pattern.
All four species demonstrated short term (weekly and monthly) utilization of a seasonal range that was more or less consistent, with some minor variations (Fig. 3). Cattle, deer and pigs, for example, used less than half (45%, 37%, 43%, respectively) of their 4-month range within 1 week, while possums used 62% of their 4-month range within 1 week and 85% of it within 4 weeks.
In addition to weekly and monthly analyses for proportional utilization of seasonal range, we also had sufficient data to assess daily range utilization, but for possums only: on average, possum daily ranges were 6·0 ha (range 0·8–18·8 ha, n = 20), equivalent to 26% (range 11–66%) of the possums' 4-monthly home-range size.
DISCUSSION
Home-range size
This study permitted, for the first time, a direct comparison of home-range utilization by the five main New Zealand hosts of bovine TB that is not confounded by major differences in habitat type. The findings are generally consistent with previous studies of wildlife in New Zealand, with possums having small home ranges (generally <30 ha), and red deer, pigs, and ferrets having ranges 1–2 orders of magnitude larger. In our study, the all-male cattle covered an average range of over 30 km2 in this mountainous dry grass/scrubland habitat, a value somewhat higher than previous reports of range movements for free-ranging cattle grazing high-altitude meadows and forest margins in the western United States (e.g. 0·7–3·3 km2 [32] and 9·2 km2 [33]). In common with cattle, the home-range sizes we recorded here for wild red deer (38 km2) are towards the upper extreme of values reported previously. They are more akin to the large ranges reported for deer in old-growth forest in Poland (adult male ranges of 36·0 km2, adult females 8·4 km2 [34]) than to those in largely unforested Scottish Highlands (which are ostensibly similar to our dry grass/scrubland habitat), where females' home ranges average between 100 ha and 500 ha [35] or in the Bavarian Alps where seasonal range sizes of 65–171 ha were recorded for non-migratory female deer [36]. Similarly, the possum range sizes recorded in this study (average 22·3 ha by GPS monitoring) are larger than the typical 1–5 ha ranges observed in more favourable habitats such as broadleaved forests, but are similar to those of possums in semi-arid land with sparse vegetation elsewhere in New Zealand [10, 19]. In contrast, the pigs in this study occupied areas of only a few hundred hectares, whereas pigs monitored in tussock grassland/scrub habitat in the Pisa Range (Central Otago, South Island of New Zealand) varied their habitat coverage between 550 ha and 15 700 ha [37]. Pigs can utilize relatively small areas when food and cover are adequate, such as the range sizes of just 28–209 ha recorded for pigs in rough pasture/beech forest habitat at Murchison, ∼100 km northwest of our study area [38]. Ferrets in this study covered an average range size of 104 ha, and had the largest disparity in range sizes between the sexes of any of the five species studied (males covered, on average, 3·5 times the area covered by females). Ferret home-range sizes in New Zealand have previously been recorded between 12 ha and >200 ha [39], with larger ranges in semi-arid pastureland holding moderate to high densities of rabbits as a major prey source.
Temporal utilization of home ranges in relation to TB risk
This study was prompted primarily by the need to help refine a new approach to wildlife disease surveillance in New Zealand to determine whether intensive culling of possum populations has successfully eliminated TB from possum populations so that control can be stopped [9]. This new approach [17, 18] is based on spatially explicit modelling of TB detection probabilities, using either sentinel species, possums themselves, or possum surveillance devices. However, the home-range data available to parameterize the TB detection kernel models are suboptimal in terms of there being few data for some species (particularly pigs, deer and cattle), and with the relative differences in range size between species being confounded by the data being from different habitats.
In general, animal-ranging studies usually report movement data on the basis of annual range size. However, in the context of defining disease detection kernels in susceptible TB host species, the relevant home-range size estimate should, intuitively, be related to the time-frame over which a sentinel is at risk of becoming infected (in the New Zealand case, by transmission from possums). Possums with clinical disease and externally detectable TB symptoms survive for only about 4 months, on average [40, 41]; further, recent research [42] has shown that very few artificially infected possums survive for more than 6 months, and that the period when a tuberculous possum is most likely to be exhibiting gross tuberculous pathology (and is therefore most likely to be infectious to other animals) is unlikely to be longer than ∼4 months. On this basis, we suggest that a 4-month home-range size estimate would be the most appropriate parameter for setting detection kernel size when possums are used as sentinels for detecting TB in other possums.
That logic can be extended to the other possum/TB detection systems currently being employed, namely leg-hold trapping for monitoring possums and interference devices for monitoring possum activity (Chew cards [43]). For traps, the possum detection probabilities that underpin the TB detection kernels derived from them are based on the probability of capture of an individual possum per trap per night [44], but in practice these traps are set for three consecutive nights. Chew cards are usually set for a week-long period in the field as surveillance devices. These detection systems therefore provide insight into where a TB possum might have been during the course of 3–7 days. As our results indicate that the weekly home-range sizes of possums average only about 60% of their 4-month range sizes, this suggests that using the latter as the basis for detection kernel estimation could overstate the TB surveillance coverage provided by the device. Countering that, however, the estimates of detection probability currently used in surveillance-based modelling tend to have been derived using home-range values based on longer-period estimates – for example, Ball et al. [44] have reported a 5-month possum range size for this purpose. This automatically compensates for the bias, in that although the coverage provided by an individual trap may be overstated, the area-wide estimate of TB detection probability remains unbiased.
With regard to other sentinel species, the period over which a TB spillover host may become infected varies according to how transmission occurs. For pigs and ferrets, M. bovis transmission is likely to occur by the scavenging of carcasses of dead tuberculous possums [6, 11]. The period of infectiousness of the carcass is likely to be temperature-dependent, since environmental conditions affect the survival of M. bovis bacilli in tissues [45]. M. bovis viability in a carcass may be as short as a few days in the height of summer, when carcass decomposition is usually rapid, or up to 2–3 months in the depths of winter, when (in some places) possum carcasses may remain frozen for weeks. We suggest for scavenging species a monthly ranging estimate is probably the most appropriate measure of risk-related home-range size to use in TB detection modelling. Our results for pigs suggest, for the present study area at least, a home-range size of about 1·7 km2 is appropriate for that species. For deer and cattle, transmission of M. bovis is believed to occur most frequently via their direct interaction with either moribund possums in the short terminal stages of disease, or with recently dead animals, via inquisitiveness [46, 47]; these are both periods when tuberculous possums are likely to be shedding M. bovis bacilli via draining sinus tracts from necrotic peripheral lymph nodes [42, 48], which is a common feature of clinical TB in possums [49, 50]. If the window of possum–deer/cattle infectiousness is indeed only a few days either side of possum death, we suggest that if these species are used as sentinels, then a weekly range size is likely to be the most appropriate measure of their spatial coverage as TB sentinels – equating to approximately 9 km2 for the free ranging cattle and 11 km2 for the deer in our study.
In summary, we argue that the period during which the presence of a tuberculous possum can be detected (either by direct surveillance of possums using detection-and-capture devices, or by indirect surveillance using spillover hosts as sentinels) is always likely to be much shorter than a year, and that the length of this detection window is likely to vary widely between species. The spatial scale of the surveillance coverage provided by the different sentinel species will therefore depend not only on differences in their long-term home-range sizes, but on how much of the estimated total range is used on a weekly or monthly basis (which our results show can often be only half to three quarters as large). Moreover, future estimates of TB detection probabilities for possum capture/detection devices should be calculated using the appropriate short-term home-range size, which should enable more accurate representation of surveillance coverage.
Supplementary Material
Supplementary information supplied by authors.
ACKNOWLEDGEMENTS
We thank Landcare Research field staff for excellent technical assistance throughout these studies. We also thank Dr Phil Cowan and Dr Dean Anderson (Landcare Research) for reviewing an earlier draft of this manuscript. The protocols followed in this research were approved by the Animal Ethics Committee of Landcare Research. This work was funded by the Animal Health Board in New Zealand.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268813000289.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.O'Reilly LM, Daborn CJ. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tubercle and Lung Disease 1995; 76 (Suppl. 1): 1–46. [DOI] [PubMed] [Google Scholar]
- 2.Good M, Duignan A. Perspectives on the history of bovine TB and the role of tuberculin in bovine TB eradication. Veterinary Medicine International 2011. Article ID 410470. doi: 10.4061/2011/410470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman JD, Cooke MM. Mycobacterium bovis infection in wildlife in New Zealand. Tuberculosis 2001; 81: 191–202. [DOI] [PubMed] [Google Scholar]
- 4.Morris RS, Pfeiffer DU. Directions and issues in bovine tuberculosis epidemiology and control in New Zealand. New Zealand Veterinary Journal 1995; 43: 256–265. [DOI] [PubMed] [Google Scholar]
- 5.Nugent G. Maintenance, spillover and spillback transmission of bovine tuberculosis in multi-host wildlife complexes: a New Zealand case study. Veterinary Microbiology 2011; 5: 151: 34–42. [DOI] [PubMed] [Google Scholar]
- 6.Caley P, Hone J. Disease transmission between and within species, and the implications for disease control. Journal of Applied Ecology 2004; 41: 94–104. [Google Scholar]
- 7.Nugent G, Yockney IJ, Whitford EJ. Intraspecific transmission of Mycobacterium bovis among penned feral pigs in New Zealand. Journal of Wildlife Diseases 2011; 47: 364–372. [DOI] [PubMed] [Google Scholar]
- 8.Animal Health Board. Animal Health Board Annual Report 2011. Wellington, New Zealand, pp. 1–67. [Google Scholar]
- 9.Hutchings S, Hancox N, Livingstone P. Approaches to eradication of tuberculosis from wildlife in New Zealand: a revised pest management strategy. Vetscript 2011; 24: 8–12. [Google Scholar]
- 10.Cowan PE, Clout M. Possums on the move: activity patterns, home ranges, and dispersal. In: Montague TL (ed.). The Brushtail Possum: Biology, Impact, and Management of an Introduced Marsupial. Lincoln, New Zealand: Manaaki Whenua Press, 2000, pp. 24–34. [Google Scholar]
- 11.Nugent G, Whitford J, Young N. Use of released pigs as sentinels for Mycobacterium bovis. Journal of Wildlife Diseases 2002; 38: 665–677. [DOI] [PubMed] [Google Scholar]
- 12.Nugent G. Deer and pigs as hosts of bovine tuberculosis, and their potential use as sentinels of disease presence. Proceedings of the New Zealand Society of Animal Production 2001; 61: 64–67. [Google Scholar]
- 13.de Lisle GW, et al. Surveillance of wildlife for Mycobacterium bovis infection using culture of pooled tissue samples from ferrets (Mustela furo). New Zealand Veterinary Journal 1995; 53: 14–18. [DOI] [PubMed] [Google Scholar]
- 14.Sangster C, et al. Feasibility of using coyotes (Canis latrans) as sentinels for bovine mycobacteriosis (Mycobacterium bovis) infection in wild cervids in and around Riding Mountain National Park, Manitoba, Canada. Journal of Wildlife Diseases 2007; 43: 432–438. [DOI] [PubMed] [Google Scholar]
- 15.VerCauteren KC, et al. Surveillance of coyotes to detect bovine tuberculosis, Michigan. Emerging Infectious Diseases 2008; 14: 1862–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berentsen AR, et al. Active use of coyotes (Canis latrans) to detect bovine tuberculosis in northeastern Michigan, USA. Veterinary Microbiology 2011; 151: 126–132. [DOI] [PubMed] [Google Scholar]
- 17.Anderson DP, et al. A novel approach to assess the probability of disease eradication from a wild animal reservoir host. Epidemiology and Infection. Published online: 13 February 2013. doi: 10.1017/S095026881200310X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosson MAJ, et al. Proving freedom in a disease with multiple host species: an area case study for TB control in New Zealand. Epidemiologie et Sante Animale 2011; 59–60: 95–97. [Google Scholar]
- 19.Glen AS, et al. Ecology of brushtail possums in a New Zealand dryland ecosystem. New Zealand Journal of Ecology 2012; 36: 29–37. [Google Scholar]
- 20.Byrom AE, et al. Cost effective control of Tb in the northern South Island high country: identifying the habitats and vector species requiring control. Landcare Research contract report LC0708/110; Manaaki Whenua – Landcare Research, 2008, Lincoln, New Zealand, pp. 1–79. [Google Scholar]
- 21.Caley P, Morriss GM. Summer/autumn movements, mortality rates and density of feral ferrets (Mustela furo) at a farmland site in North Canterbury, New Zealand. New Zealand Journal of Ecology 2001; 25: 53–60. [Google Scholar]
- 22.Nugent G, Whitford J. Animal Health Board Project No. R-10652 Relative Utility of Tb Hosts as Sentinels for Detecting Tb. Landcare Research contract report; 0708/032, 2007, Manaaki Whenua – Landcare Research, Lincoln, New Zealand, pp. 1–38. [Google Scholar]
- 23.Recio MR, et al. Lightweight GPS-tags, one giant leap for wildlife tracking? An assessment approach. PLoS ONE 2011; 6: e28225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Eon RG, Delparte D. Effects of radio-collar position and orientation on GPS radio-collar performance, and the implications of PDOP in data screening. Journal of Applied Ecology 2005; 42: 383–388. [Google Scholar]
- 25.Lewis JS, et al. Effects of habitat on GPS collar performance: using data screening to reduce location error. Journal of Applied Ecology 2007; 44: 663–671. [Google Scholar]
- 26.Rodgers AR, et al. HRT: home range tools for ArcGIS, version 1.1. Ontario Ministry of Natural Resources, 2011, Centre for Northern Forest Ecosystem Research, Thunder Bay, Ontario, Canada. [Google Scholar]
- 27.Seaman DE, Powell RA. An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology 1996; 77: 2075–2085. [Google Scholar]
- 28.Gitzen RA, Millspaugh JJ, Kernohan BJ. Bandwidth selection for fixed-kernel analysis of animal utilisation distributions. Journal of Wildlife Management 2006; 70: 1334–1344. [Google Scholar]
- 29.ESRI. ArcGIS: release 9.3 edition [computer program]. Environmental Systems Research Institute (ESRI), 2008, Redlands, California. [Google Scholar]
- 30.Seaman DE, et al. Effects of sample size on kernel home range estimates. Journal of Wildlife Management 1999; 63: 739–747. [Google Scholar]
- 31.Börger L, et al. Effects of sampling regime on the mean and variance of home range size estimates. Journal of Animal Ecology 2006; 75: 1393–1405. [DOI] [PubMed] [Google Scholar]
- 32.Howery LD, et al. Differences in home range and habitat use among individuals in a cattle herd. Applied Animal Behaviour Science 1996; 49: 305–320. [Google Scholar]
- 33.Roath VHF, Krueger WC. Cattle grazing and behaviour on a forested range. Journal of Range Management 1982; 35: 332–342. [Google Scholar]
- 34.Kamler JF, Jędrzejewska B, Jędrzejewski W. Factors affecting daily ranges of red deer Cervus elaphus in Białowieża Primeval Forest, Poland. Acta Theriologica 2007; 52: 113–118. [Google Scholar]
- 35.Clutton-Brock TH, Albon SD. Red Deer in the Highlands. Oxford: BSP Professional Books, 1989. [Google Scholar]
- 36.Georgii B, Schroder W. Home range and activity patterns of male red deer (Cervus elaphus L.) in the Alps. Oecologia (Berlin) 1983; 58: 238–248. [DOI] [PubMed] [Google Scholar]
- 37.Knowles GJE. Use of the Judas pig methodology for controlling tuberculosis in feral pigs. MAF Quality Management Contract Report 73/90. Animal Health Board, Wellington, New Zealand, 1994, pp. 1–32. [Google Scholar]
- 38.McIlroy JC. Aspects of the ecology of feral pigs (Sus scrofa) in the Murchison area, New Zealand. New Zealand Journal of Ecology 1989; 12: 11–22. [Google Scholar]
- 39.Norbury GL, Norbury DC, Heyward RP. Space use and denning behaviour of wild ferrets (Mustela furo) and cats (Felis catus). New Zealand Journal of Ecology 1998; 22: 149–159. [Google Scholar]
- 40.Ramsey D, Cowan P. Mortality rate and movements of brushtail possums with clinical tuberculosis (Mycobacterium bovis) infection. New Zealand Veterinary Journal 2003; 51: 179–185. [DOI] [PubMed] [Google Scholar]
- 41.Norton S, Corner LA, Morris RS. Ranging behaviour and duration of survival of wild brushtail possums (Trichosurus vulpecula) infected with Mycobacterium bovis. New Zealand Veterinary Journal 2005; 53: 293–300. [DOI] [PubMed] [Google Scholar]
- 42.Nugent G, et al. Mortality rate and development of gross pathology due to tuberculosis in wild brushtail possums (Trichosurus vulpecula) following low dose subcutaneous injection of Mycobacterium bovis. Preventive Veterinary Medicine. Published online: 10 October 2012. doi: 10.1016/j.prevetmed.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Sweetapple P, Nugent G. Chew-track-cards: a multiple-species small mammal detection device. New Zealand Journal of Ecology 2011; 35: 153–162. [Google Scholar]
- 44.Ball SJ, et al. A method for estimating wildlife detection probabilities in relation to home range use: insights from a field study on the brushtail possum (Trichosurus vulpecula). Wildlife Research 2005; 32: 217–227. [Google Scholar]
- 45.Barron MC, et al. Longevity of Mycobacterium bovis in brushtail possum (Trichosurus vulpecula) carcasses, and contact rates between possums and carcasses. New Zealand Veterinary Journal 2011; 59: 209–217. [DOI] [PubMed] [Google Scholar]
- 46.Paterson BM, Morris RS. Interactions between beef cattle and simulated tuberculous possums on pasture. New Zealand Veterinary Journal 1995; 43: 289–293. [DOI] [PubMed] [Google Scholar]
- 47.Sauter CM, Morris RS. Behavioural studies on the potential for direct transmission of tuberculosis from feral ferrets (Mustela furo) and possums (Trichosurus vulpecula) to farmed livestock. New Zealand Veterinary Journal 1995; 43: 294–300. [DOI] [PubMed] [Google Scholar]
- 48.Nugent G, et al. Percutaneous interdigital injection of Mycobacterium bovis as a model for tuberculous lesion development in wild brushtail possums, Trichosurus vulpecula. Journal of Comparative Pathology 2012; 148: 33–42. [DOI] [PubMed] [Google Scholar]
- 49.Coleman JD. Distribution, prevalence, and epidemiology of bovine tuberculosis in brushtail possums, Trichosurus vulpecula, in the Hohonu Range, New Zealand. Australian Wildlife Research 1988; 15: 651–663. [Google Scholar]
- 50.Cooke MM, et al. Naturally occurring tuberculosis caused by Mycobacterium bovis in brushtail possums (Trichosurus vulpecula): II. Pathology. New Zealand Veterinary Journal 1995; 43: 315–321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information supplied by authors.
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268813000289.
click here to view supplementary material