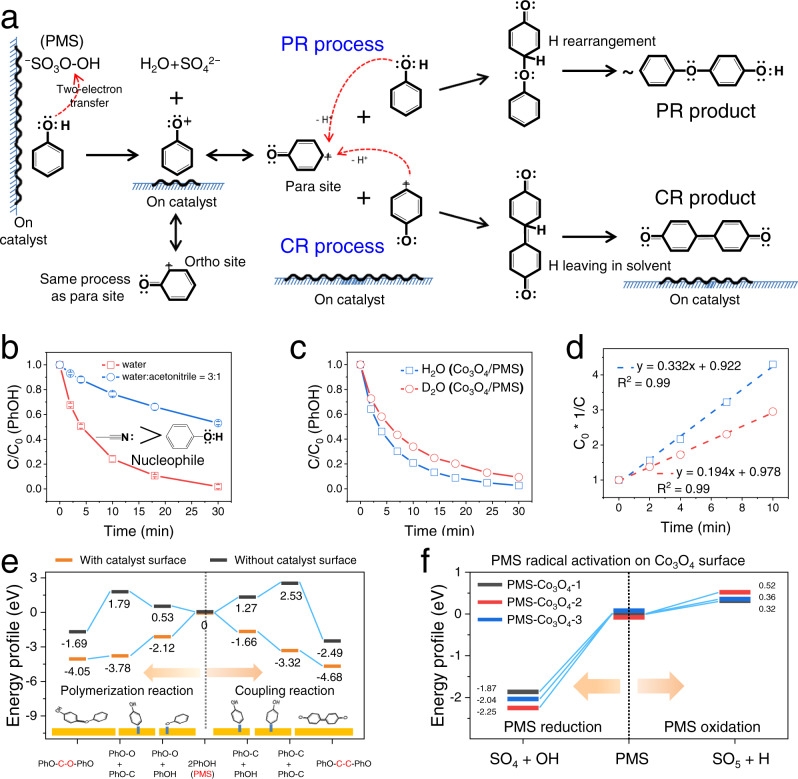
Fig. 5. Evolution process of DOTP and thermodynamic feasibility analysis.
a Proposed elementary reaction pathways of the oxidative coupling and polymerization of PhOH on the catalyst surface in the DOTP. b Competitive inhibition of acetonitrile on PhOH removal. c Removal processes of PhOH in H2O and D2O. d Corresponding kinetic curves of c. The KIE value was calculated to be KH/KD ~1.4, which is in the range of secondary kinetic isotope effects. e Thermodynamic potential energy curves during the reaction in DOTP. f Thermodynamic potential energy curves of the reductive and oxidative activation processes of PMS on the Co3O4 surface in the AOP. PR polymerization reaction, CR coupling reaction.