SUMMARY
Haemorrhagic fever with renal syndrome (HFRS) is a type of vector-borne zoonosis sensitive to climate change. To explore the short-term effect of air temperature and amount of precipitation on HFRS incidence, a total of 13 722 clinically confirmed HFRS cases from January 1977 to December 2001 in Junan County, China were included in this study. According to symmetric bidirectional case-crossover design, the hazard period (the three calendar months preceding the month when the case was diagnosed) and the control period (the same calendar month of the year before and the year after the hazard period) matched and conditional logistic regression was used to examine the effect of monthly mean temperature and precipitation on the risk of HFRS. The results showed the facilitating climatic conditions for HFRS included: condition with moderate mean air temperature (10–25 °C) and abundant precipitation (>120 mm) 3 months before [odds ratio (OR) 1·346, 95% confidence interval (CI) 1·191–1·522] and 2 months before (OR 1·193, 95% CI 1·063–1·339); and condition with temperature >25 °C and abundant precipitation (>120 mm) 3 months before (OR 1·17, 95% CI 1·004–1·363). Temperature of 10–25 °C and moderate precipitation (10–120 mm) in the current month was the most favourable condition for HFRS incidence.
Key words: Amount of precipitation, atmospheric temperature, case-crossover study, haemorrhagic fever with renal syndrome
INTRODUCTION
Haemorrhagic fever with renal syndrome (HFRS) is a zoonosis caused by different species of Hantavirus, characterized by high fever and varying degrees of renal insufficiency and haemorrhage. It has been reported throughout Europe and Asia with 90% of cases occurring in China, where HFRS is an extremely dangerous viral disease second only to viral hepatitis [1]. The annual incidence of HFRS in China peaked at 11·06 new cases/100 000 persons in 1986, declined in the late 1990s, but has rebounded in recent years. It remains a public health problem with 20 000–50 000 human cases annually in mainland China [2, 3]. HFRS incidence distinctly shows spatial clustering and temporal variability [4, 5]. For example, annualized average incidence at the county-level ranged from 0 to 122·117/100 000 during 1994–1998 [4]. In the past decades, HFRS has occurred in about 10-year endemic cycles in east and northeast China [6].
Various rodent species are natural reservoir hosts and serve as sources of hantavirus infection. In China, HFRS is predominantly caused by Hantaan virus and Seoul virus, for which Apodemus agrarius and Rattus norvegicus are the leading rodent hosts, respectively [2]. Transmission of hantaviruses from rodents to humans is usually through inhalation of aerosols generated by contaminated excreta, saliva, or urine and possibly through contaminated food or rodent bites [1, 3, 7]. Factors related to rodent habitats, breeding and survival – such as natural environment including elevation, soil type, vegetation, climate change and human behaviours including farming, cultivating and migration – can influence the transmission of hantaviruses and the epidemic process of HFRS [8]. Large populations of rodents can potentially increase the transmission of hantaviruses to humans [9, 10]. Excess proliferation of rodent populations related to climate change is of considerable international public health concern [11, 12]. Previous studies have shown that HFRS epidemics are sensitive to climatic conditions [9, 12, 13]. However, the quantitative relationship between climate variation and HFRS incidence remains to be determined, especially in high-incidence areas.
A case-crossover design was proposed as an alternative to spatial-ecological modelling to make causal inferences in environmental epidemiology [14]. It is a method for studying the effect of a risk factor on a health event using only cases with the idea of comparing a case's exposure immediately prior to or during the case-defining event with that same person's exposure at otherwise similar ‘reference’ times [15]. Each case's exposure values comprise a matched set so that conditional logistic regression is typically used to estimate an odds ratio as the measure of association [16]. Case-crossover design has the advantage of controlling time-invariant factors because it only involves cases and each case is compared to him/herself [17].
This study was aimed at exploring the association of atmosphere temperature and precipitation with HFRS incidence in Junan County of Shandong Province, a region with a high prevalence of HFRS, by the method of case-crossover analysis.
MATERIALS AND METHODS
Study site
Junan County is located in the southeast of Shandong Province and on the border with Jiangsu Province, and has a population of about 970 000 people and a total area of 1752 km2. It has a warm temperate and semi-humid continental climate with four distinct seasons. The average altitude of this county is 200 m (range 19·9–662·2 m) above sea level. The county reported the highest epidemic strength of HFRS in Shandong Province with an annual average incidence reaching 55·20/100 000 over the past decades and therefore it was selected as one of the 39 national monitoring spots of HFRS.
The data of the HFRS cases for this study were from the National Notifiable Disease Surveillance System. Monthly climatic data of this county were obtained from the Meteorological Information Center of Shandong Province.
HFRS surveillance in Junan County
The HFRS surveillance system has been established in Junan County since 1975 to examine the general situation of this disease, to monitor its temporal trend, and to assess the effectiveness of interventions. HFRS case diagnosis and reporting follow the national protocols proposed by the Ministry of Health [18]. Briefly, healthcare providers and clinics are required to report clinically diagnosed/suspected cases of HFRS to the county's Center for Disease Control and Prevention. Upon receipt of a case report, trained health professionals check the report and review medical records to confirm the clinical diagnosis. Blood samples from the case patient might be collected and a positive serum test of specific antibody to Hantavirus was used to confirm the disease by identifying the Hantavirus type.
Case-crossover design
The latent period from hantavirus infection to the onset of clinical symptoms ranges from 4 to 45 days, usually 2–4 weeks [18]. It usually takes about 3 months for rodents, the reservoirs of hantaviruses, to reach the adult stage of life from birth [19, 20]. Therefore, we assumed that for a case the hazard period was during the most recent 4 months, just before the onset of disease. We defined an exposure window as a time interval in which the climatic condition is assessed as a potential risk factor for HFRS. In accordance with the available climatic data for calendar month, we defined four case exposure windows: the calendar month when the case was diagnosed (exposure window 0), and each of the three calendar months preceding the month when the case was diagnosed (exposure windows -1, -2, -3, respectively). For example, for a HFRS case diagnosed in November 1985, the case exposure windows -3, -2, -1 and 0 would be August, September, October and November 1985, respectively. Symmetric bidirectional case-crossover design [16] was used in this study in order to control for confounding due to possible secular trend that was not related to the climatic conditions of interest. Each case exposure window was matched with two referent exposure windows symmetric to the case exposure window, one a year before and the other a year after the case exposure window. For example, if the case exposure window was November 1985, then the referent exposure windows were November 1984 and November 1986. A case exposure window and the corresponding two referent exposure windows resulted in a 1:2 match set analogous to a case-control study.
Data analyses
Because the effect of climatic variables on the incidence of diseases is not linear [21], air temperature and precipitation were transformed to categorical variables. The mean temperature for each month was grouped into three levels: low (<10 °C), moderate (10–25 °C), and high (>25 °C). The monthly amount of precipitation was grouped into <10 mm, 10–120 mm, and >120 mm. Such categorization of temperature and precipitation was based on the reports of the climatic conditions thought to be suitable for rodents' breeding [22, 23].
The climatic conditions of the exposure windows were compared with the climatic conditions of the referent windows with the application of conditional logistic regression. The odds ratio (OR) of HFRS and its 95% confidence interval (CI) for a climatic condition compared to the referent condition were estimated. All the data analyses were performed using SAS v. 9.13 (SAS Institute Inc., USA).
RESULTS
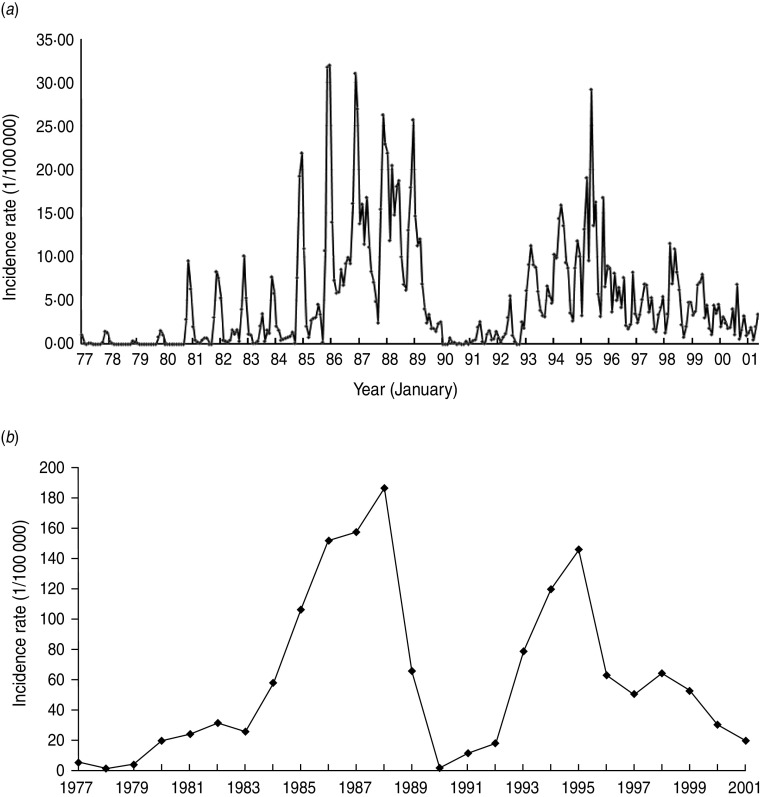
There were 13 722 clinically confirmed cases of HFRS reported to Junan Center for Disease Control and Prevention from January 1977 to December 2001. The yearly incidence varied from 1·59 to 185·93/100 000 with a mean of 61·72/100 000. The monthly incidence during the period is shown in Figure 1.
Fig. 1.
(a) Monthly haemorrhagic fever with renal syndrome (HFRS) incidence (1/100 000) in Junan County from January 1977 to December 2001. (b) Annual HFRS incidence rate (1/100 000) in Junan County from 1977 to 2001.
According to the meteorological data, the annual average temperature and accumulative precipitation for the period from 1976 to 2002 were 13·24 °C and 787·99 mm, respectively. January had the lowest temperature with a mean temperature of −0·99 °C and July was the warmest month with a mean monthly temperature of 25·79 °C. Average monthly precipitation from 1976 to 2002 varied from the lowest of 10·35 mm in December to the highest of 226·32 mm in July (Table 1).
Table 1.
Mean temperature and precipitation by calendar month from 1976 to 2002 in Junan County, China
| Calendar month | Mean temperature (°C) | Precipitation (mm) | Mean humidity (%) |
|---|---|---|---|
| January | −0·99 | 15·78 | 62·20 |
| February | 1·30 | 15·74 | 61·54 |
| March | 6·23 | 23·23 | 61·25 |
| April | 13·25 | 32·80 | 60·96 |
| May | 19·05 | 60·57 | 65·23 |
| June | 23·05 | 97·86 | 72·56 |
| July | 25·79 | 226·32 | 83·27 |
| August | 25·35 | 156·16 | 82·40 |
| September | 21·03 | 72·34 | 74·23 |
| October | 15·16 | 42·93 | 69·21 |
| November | 7·64 | 25·60 | 66·01 |
| December | 1·24 | 10·35 | 64·42 |
| Year-round | 13·24 | 779·68 | 68·67 |
Atmosphere temperature and HFRS risk
The relationship between temperature and HFRS risk is shown in Table 2. During the previous 3 months of a HFRS case being diagnosed, a monthly mean temperature level >25 °C, compared to the monthly mean temperature of 10–25 °C, was significantly associated with a lower risk of HFRS at any exposure window consistently. The estimations of ORs were 0·802 (95% CI 0·672–0·958), 0·289 (95% CI 0·233–0·358), and 0·662 (95% CI 0·563–0·779) for exposure windows 0, -1 and -2, respectively. In contrast, there is no consistent relationship of a temperature <10 °C with HFRS risk over the different exposure windows. The cold weather with mean temperature <10 °C only at exposure window -2 (2 months before the occurrence of HFRS cases) significantly decreased the number of cases (OR 0·339, 95% CI 0·183–0·629). The mean temperature of exposure window -3 (3 months before the occurrence of HFRS cases) was not found to be associated with HFRS incidence (P = 0·4428 and 0·1421 for cold weather and hot weather, respectively).
Table 2.
Monthly mean temperature levels at different exposure windows and HFRS risk: results of conditional logistic regression
| Exposure window* | Mean air temperature (°C) | Number of HFRS cases | OR | (95% CI) | P value | ||
|---|---|---|---|---|---|---|---|
| Hazard period | Control period 1† | Control period 2‡ | |||||
| 0 | 10–25 | 5394 | 5355 | 5334 | 1·000 | — | — |
| <10 | 7221 | 7233 | 7227 | 0·725 | (0·462–1·138) | 0·1623 | |
| >25 | 1107 | 1134 | 1161 | 0·802 | (0·672–0·958) | 0·0147 | |
| -1 | 10–25 | 6814 | 6656 | 6650 | 1·000 | — | — |
| <10 | 6345 | 6347 | 6339 | 1·064 | (0·697–1·624) | 0·7744 | |
| >25 | 563 | 719 | 733 | 0·289 | (0·233–0·358) | <0·0001 | |
| -2 | 10–25 | 6882 | 6757 | 6793 | 1·000 | — | — |
| <10 | 5840 | 5853 | 5860 | 0·339 | (0·18–0·629) | 0·0006 | |
| >25 | 1000 | 1112 | 1069 | 0·662 | (0·563–0·779) | <0·0001 | |
| -3 | 10–25 | 5929 | 5852 | 5918 | 1·000 | — | — |
| <10 | 5662 | 5668 | 5665 | 0·823 | (0·501–1·353) | 0·4428 | |
| >25 | 2131 | 2202 | 2139 | 0·922 | (0·826–1·028) | 0·1421 | |
HFRS, Haemorrhagic fever with renal syndrome; OR, odds ratio; CI, confidence interval.
Exposure windows 0, -1, -2, -3 represent the calendar month when the case was diagnosed and the three calendar months preceding the month when the case was diagnosed.
The same calendar month of the year before the hazard period.
The same calendar month of the year after the hazard period.
Amount of precipitation and HFRS risk
The results of conditional logistic regressions of HFRS regarding the amount of precipitation assessed by the different exposure windows are shown in Table 3. Lack of precipitation (monthly precipitation <10 mm) and excessive precipitation (monthly precipitation >120 mm) in the month that the case occurred were both associated with lower risk of HFRS compared to a moderate amount of precipitation (monthly precipitation between 10 and 120 mm). The estimated ORs were 0·821 (95% CI 0·774–0·871) and 0·860 (95% CI 0·774–0·955), respectively. Other significant associations included excessive precipitation at exposure windows -2 and -3 with increasing risk of HFRS (OR 1·355, 95% CI 1·210–1·472 and OR 1·217, 95% CI 1·108–1·336, respectively), which means that plentiful rain during the preceding 2–3 months would be a risk factor for an HFRS epidemic.
Table 3.
Monthly precipitation levels at different exposure windows and HFRS risk: results of conditional logistic regression
| Exposure window* | Precipitation (mm) | Number of HFRS cases | OR | (95% CI) | P value | ||
|---|---|---|---|---|---|---|---|
| Hazard period | Control period 1† | Control period 2‡ | |||||
| 0 | 10–120 | 8210 | 7828 | 7812 | 1·000 | — | — |
| <10 | 3772 | 3992 | 4196 | 0·821 | (0·774–0·871) | <0·0001 | |
| >120 | 1740 | 1902 | 1714 | 0·860 | (0·774–0·955) | 0·0049 | |
| -1 | 10–120 | 8629 | 8849 | 8513 | 1·000 | — | — |
| <10 | 3711 | 3526 | 3817 | 1·024 | (0·965–1·087) | 0·3990 | |
| >120 | 1382 | 1347 | 1392 | 1·027 | (0·926–1·140) | 0·6124 | |
| -2 | 10–120 | 8843 | 8956 | 8736 | 1·000 | — | — |
| <10 | 3191 | 3173 | 3574 | 0·889 | (0·835–0·947) | 0·0007 | |
| >120 | 1688 | 1593 | 1412 | 1·335 | (1·210–1·472) | <0·0001 | |
| -3 | 10–120 | 8031 | 8354 | 7886 | 1·000 | — | — |
| <10 | 3128 | 2872 | 3468 | 0·981 | (0·919–1·048) | 0·5711 | |
| >120 | 2563 | 2496 | 2368 | 1·215 | (1·107–1·334) | <0·0001 | |
HFRS, Haemorrhagic fever with renal syndrome; OR, odds ratio; CI, confidence interval.
Exposure windows 0, -1, -2, -3 represent the calendar month when the case was diagnosed and the three calendar months preceding the month when the case was diagnosed.
The same calendar month of the year before the hazard period.
The same calendar month of the year after the hazard period.
Combinations of temperature and precipitation categories and HFRS risk
In addition to the independent analysis for temperature and precipitation (Tables 2 and 3), we analysed how the weather conditions of cross-classification by temperature and precipitation affected the incidence of HFRS using conditional logistic regressions. There were eight cross-groups, excluding the hot and dry weather condition (mean temperature >25 °C and monthly precipitation <10 mm) classified since no month during the study period had hot and dry weather. The condition with appropriate temperature (10–25 °C) and moderate precipitation (10–120 mm) was treated as the referent group, odds ratios and their confidence intervals are displayed for other conditions of the four respective exposure windows (see Table 4). This shows that compared to the referent climatic condition, any combination with mean temperature and monthly precipitation beyond its appropriate range in the preceding 2 months (exposure windows 0 and -1) tended to be associated with a lower risk of HFRS (all ORs <1), which means that appropriate temperature and moderate precipitation is the most favourable condition for occurrence of HFRS. On the contrary, abundant precipitation combined with appropriate air temperature 2 or 3 months before was a risk factor for HFRS. The estimated ORs of precipitation >120 mm with mean temperature between 10 °C and 25 °C were 1·193 (95% CI 1·063–1·339) and 1·348 (95% CI 1·193–1·524) at exposure windows -2 and -3, respectively. The estimated OR of precipitation >120 mm with mean temperature >25 °C was 1·171 (95% CI 1·005–1·334).
Table 4.
Associations of weather conditions and HFRS incidence: results of conditional logistic regression
| Exposure window | Mean temperature (°C) | Monthly precipitation (mm) | OR | (95% CI) | P value |
|---|---|---|---|---|---|
| 0 | 10–25 | 10–120 | 1·000 | — | — |
| 10–25 | <10 | 0·569 | (0·499–0·647) | <0·0001 | |
| 10–25 | >120 | 0·734 | (0·649–0·83) | <0·0001 | |
| <10 | 10–120 | 0·427 | (0·267–0·684) | 0·0004 | |
| <10 | <10 | 0·383 | (0·239–0·613) | <0·0001 | |
| <10 | >120 | 0·275 | (0·151–0·502) | <0·0001 | |
| >25 | 10–120 | 0·402 | (0·302–0·534) | <0·0001 | |
| >25 | >120 | 0·724 | (0·591–0·888) | 0·0019 | |
| -1 | 10–25 | 10–120 | 1·000 | — | — |
| 10–25 | <10 | 0·636 | (0·561–0·721) | <0·0001 | |
| 10–25 | >120 | 0·929 | (0·826–1·044) | 0·2135 | |
| <10 | 10–120 | 0·640 | (0·411–0·996) | 0·0479 | |
| <10 | <10 | 0·761 | (0·488–1·186) | 0·2279 | |
| <10 | >120 | 0·503 | (0·275–0·918) | 0·0251 | |
| >25 | 10–120 | 0·125 | (0·087–0·181) | <0·0001 | |
| >25 | >120 | 0·316 | (0·249–0·402) | <0·0001 | |
| -2 | 10–25 | 10–120 | 1·000 | — | — |
| 10–25 | <10 | 0·954 | (0·841–1·083) | 0·4669 | |
| 10–25 | >120 | 1·193 | (1·063–1·339) | 0·0026 | |
| <10 | 10–120 | 0·341 | (0·181–0·641) | 0·0008 | |
| <10 | <10 | 0·299 | (0·159–0·563) | 0·0002 | |
| <10 | >120 | 1·073 | (0·517–2·224) | 0·8502 | |
| >25 | 10–120 | 0·599 | (0·485–0·739) | <0.0001 | |
| >25 | >120 | 0·864 | (0·708–1·054) | 0·1495 | |
| -3 | 10–25 | 10–120 | 1·000 | — | — |
| 10–25 | <10 | 0·696 | (0·594–0·815) | <0·0001 | |
| 10–25 | >120 | 1·348 | (1·193–1·524) | <0·0001 | |
| <10 | 10–120 | 0·564 | (0·335–0·951) | 0·0318 | |
| <10 | <10 | 0·590 | (0·349–0·996) | 0·0483 | |
| <10 | >120 | 0·484 | (0·267–0·881) | 0·0175 | |
| >25 | 10–120 | 1·074 | (0·916–1·259) | 0·3821 | |
| >25 | >120 | 1·171 | (1·005–1·364) | 0·0430 |
HFRS, Haemorrhagic fever with renal syndrome; OR, odds ratio; CI, confidence interval.
Bold values indicate the higher risk weather condition for HFRS incidence compared to the moderate weather condition (temperature of 10–25 °C combined with precipitation of 10–20 mm).
DISCUSSION
HFRS is epidemic in many provinces with high incidence in mainland China, although integrated intervention measures including rodent control, environment management and vaccination have been implemented for more than 10 years [24]. Geographical distribution of HFRS cases in mainland China show that HFRS mainly occurs in areas where the annual average temperature is <20 °C, the annual average relative humidity is 50–80%, and the annual total rainfall is 400–1600 mm [25]. Although the endemic areas are mainly in zones with mild temperature, HFRS is epidemic from the northernmost provinces to the southernmost provinces in east China. In contrast, there was no case reported in west China with an average altitude >2000 m, where precipitation was so rare that the relative humidity was usually <50% and rainy days were fewer than 60 each year [26]. Therefore, most researchers believe that precipitation, followed by air temperature, are the most important elements among all the influencing meteorological factors [27]. This is why we focused on these two variables in order to investigate the effects of meteorological factors on the occurrence of HFRS. Although humidity was also found to be associated with HFRS incidence in previous studies, it was not included in the present study because of the strong correlation between monthly mean humidity and monthly accumulative precipitation during the study period (r = 0·70, n = 324, P < 0·0001).
The results of our study show that the risk of HFRS cases increasing is positively associated with the amount of precipitation in the preceding 2–3 months, while moderate precipitation during the preceding 1–2 months might be necessary to facilitate the prevalence of HFRS. Plenty of rainfall is beneficial for growth of vegetation, which provides abundant food for rodents. Meanwhile, atmospheric moisture makes the food soft which encourages the rodents to consume more [27]. On the contrary, drought usually results in the number of rodents decreasing, which has a detrimental effect on the prevalence of HFRS [28]. For example, a severe drought lasting for three seasons from October 1994 in Xi'an City, China resulted in an obvious decline in HFRS incidence by 1995, falling to the lowest level in 1996 [29]. It is natural that the effect on variation of HFRS incidence lags behind climate change since the growth of vegetation, rodents' breeding and virus transmission are a series of processes. The lag period of rainfall for HFRS was about 2–3 months as shown by the results of this study. Nevertheless, excessive rain might work against an HFRS epidemic probably through curtailing the activities of rodents and humans, thereby reducing their contact opportunities. This is supported by our result that accumulative precipitation >120 mm in the same month, compared to moderate precipitation of 10–120 mm, was a disadvantage for occurrence of HFRS.
Regarding atmosphere temperature, our results show that extremes of weather (too cold or too hot) do not favour HFRS prevalence, while the most appropriate mean temperature was shown to be between 10 °C and 25 °C. It was reported that both Apodemus agrarius and Rattus norvegicus have their highest breeding rate at temperatures between 10 °C and 25 °C, while the breeding rate decreased with temperatures <10 °C or >25 °C [22, 23, 30].
The results of the combinations of temperature and precipitation suggest that the study factors appear to be associated with higher numbers of HFRS cases, which will be valuable for predicting increasing incidence. For example, abundance of precipitation >120 mm or moderate precipitation and hot weather with average temperature >25 °C might indicate an epidemic of HFRS in 3 months. Therefore, taking preventive measures in advance, e.g. deratization, avoiding contaminated food or water, intensifying individual protection would be necessary if such climatic conditions occurred.
In this study, a case-crossover design was used to analyse the effect of mean air temperature and precipitation on the risk of HFRS onset. This has advantages in controlling time-invariant confounding factors, e.g. geographical and geological factors, socioeconomic factors, and people's lifestyle [31]. Moreover, the time lag of the effect was effectively detected through setting different matching groups month by month and the nonlinear problem was avoided by defining dichotomous dummy variables in conditional logistic regressions. Compared to complex methods such as a generalized additive Poisson regression model in time-series analyses, the data analysis based on the case-crossover design is much easier to perform and the results are more explicable for epidemiologists.
Nevertheless, there are still limitations of this study. First, the long-term effect of climate on the epidemic trend of HFRS was not investigated since the case-crossover design is used to estimate the risk of a rare event associated with a short-term exposure. Second, the potential interaction effect of other meteorological variables, e.g. humidity, duration of sunshine, and atmospheric pressure was not considered. Therefore, further investigation of modelling the associations between climate and occurrence of HFRS is warranted.
CONCLUSIONS
Atmospheric temperature and amount of precipitation during the preceding 3 months can impact the endemic intensity of HFRS. The climatic conditions with relatively high risk for a HFRS epidemic included: condition with moderate mean air temperature (10–25 °C) and abundant precipitation (>120 mm) 3 months before (OR 1·348, 95% CI 1·193–1·524) or 2 months before (OR 1·193, 95% CI 1·063–1·339); condition with temperature >25 °C and abundant precipitation (>120 mm) 3 months before (OR 1·171, 95% CI 1·005–1·364); condition with appropriate temperature (10–25 °C) and moderate precipitation (10–120 mm) during the same month. A case-crossover study is an alternative method for analysing the time-series data of meteorological epidemiology.
ACKNOWLEDGEMENTS
We thank the Center for Disease Control of Junan County and the Meteorology Information Center of Shandong Province for sharing with us the data needed for this study. This study was supported by the Shandong Outstanding Young and Middle-aged Scientists Research Award Fund (2007BSB02046) and the National Basic Research Program of China (973 Program) (2012CB955500).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Shi J. Research progress of hemorrhagic fever with renal syndrome in China [in Chinese]. Chinese Journal of Zoonoses 2007; 23: 296–298. [Google Scholar]
- 2.Lei Y, et al. Landscape elements and hantaan virus-related hemorrhagic fever with renal syndrome, People's Republic of China. Emerging Infectious Diseases 2007; 13: 1301–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song G. Epidemiological progresses of hemorrhagic fever with renal syndrome in China. Chinese Medical Journal 1999; 112: 472–477. [PubMed] [Google Scholar]
- 4.Fang LQ, et al. Spatial analysis of hemorrhagic fever with renal syndrome in China. BMC Infectious Diseases 2006; 6: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin HL, et al. Analysis of the geographic distribution of HFRS in Liaoning Province between 2000 and 2005. BMC Public Health. Published online: 15 August 2007. doi: 10.1186/1471-2458-7-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song G. Epidemiological study and prevention of hemorrhagic fever with renal syndrome [in Chinese]. Chinese Journal of Public Health 2004; 20: 766–768. [Google Scholar]
- 7.Clement JP. Hantavirus. Antiviral Research 2003; 57: 121–127. [DOI] [PubMed] [Google Scholar]
- 8.Viel JF, et al. Environmental risk factors for haemorrhagic fever with renal syndrome in a French new epidemic area. Epidemiology and Infection 2011; 139: 867–874. [DOI] [PubMed] [Google Scholar]
- 9.Engelthaler DM, et al. Climatic and environmental patterns associated with hantavirus pulmonary syndrome, Four Corners region, United States. Emerging Infectious Diseases 1999; 5: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hjelle B, Glass GE. Outbreak of hantavirus infection in the Four Corners region of the United States in the wake of the 1997–1998 El Nino–Southern Oscillation. Journal of Infectious Disease 2000; 181: 1569–1573. [DOI] [PubMed] [Google Scholar]
- 11.Kausrud KL, et al. Climatically driven synchrony of gerbil populations allows large-scale plague outbreaks. Proceedings of the Royal Society of London, Series B: Biological Sciences 2007; 274: 1963–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tersago K, et al. Hantavirus disease (nephropathia epidemica) in Belgium: effects of tree seed production and climate. Epidemiology and infection 2009; 137: 250–256. [DOI] [PubMed] [Google Scholar]
- 13.Zhang WY, et al. Climate variability and hemorrhagic fever with renal syndrome transmission in northeastern China. Environmental Health Perspective 2010; 118: 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carracedo-Martinez E, et al. Case-crossover analysis of air pollution health effects: a systematic review of methodology and application. Environmental Health Perspectives 2010; 118: 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. American Journal of Epidemiology 1991; 133: 144–153. [DOI] [PubMed] [Google Scholar]
- 16.Bateson TF, Schwartz J. Control for seasonal variation and time trend in case-crossover studies of acute effects of environmental exposures. Epidemiology 1999; 10: 539–544. [PubMed] [Google Scholar]
- 17.Nitta H, et al. An introduction to epidemiologic and statistical methods useful in environmental epidemiology. Journal of Epidemiology 2010; 20: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song G. Manual of Prevention and Treatment of Hemorrhagic Fever with Renal Syndrome [in Chinese], 2nd edn. Beijing: People's Medical Publishing House, 1998, pp. 30–120. [Google Scholar]
- 19.Shi XC, Zhang YJ (eds). Murine Ecology and Murine Diseases [in Chinese]. Jinan: Shandong University Publishing House, 1991, pp. 72–73. [Google Scholar]
- 20.Liu YX, et al. Study on ecology of rodents and their medical importance in hilly areas of South Shandong [in Chinese]. Chinese Journal of Vector Biology and Control 2003; 14: 265–268. [Google Scholar]
- 21.Hu W, et al. Time-series analysis of the risk factors for haemorrhagic fever with renal syndrome: comparison of statistical models. Epidemiology and Infection 2007; 135: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang XL, et al. Study on the law of population change of Apodemus agrarius and the prevention and treatment to their damage [in Chinese]. Chinese Journal of Vector Biology and Control 1994; 5: 57–59. [Google Scholar]
- 23.Li SD, Pu WL, Mu WD. A research on the breeding pattern of the species composition of Rattus norvegicus in the farmland in central Yunnan [in Chinese]. Plant Protection Technology and Extension 1998; 18: 24–26. [Google Scholar]
- 24.Zhang Y, et al. The epidemic characteristics and preventive measures of hemorrhagic fever with renal syndrome [in Chinese]. Chinese Journal of Epidemiology 2004; 25: 466–469. [PubMed] [Google Scholar]
- 25.Fang LQ, et al. Study on the application of geographic information system in spatial distribution of hemorrhagic fever with renal syndrome in China [in Chinese]. Chinese Journal of Epidemiology 2003; 24: 265–268. [PubMed] [Google Scholar]
- 26.Bi P, et al. Climate, reservoir and occupational variables and the transmission of hemorrhagic fever with renal syndrome in China. International Journal of Epidemiology 2002; 31: 189–193. [DOI] [PubMed] [Google Scholar]
- 27.Chen ZG, et al. Meteorological factors and rats density [in Chinese]. Chinese Journal of Vector Biology and Control 2002; 13: 135. [Google Scholar]
- 28.Luo L, et al. An analysis on the relationships among the climatic indices and incidence of HFRS and the mice density [in Chinese]. Modern Preventive Medicine 2005; 32: 205–206, 222. [Google Scholar]
- 29.Yang H, Xia XC, Wu YJ. Analysis of incidence trend of hemorrhagic fever with renal syndrome in Xi'an City, 1990–2000 [in Chinese]. Literature and Information of Preventive Medicine 2002; 8: 599–600. [Google Scholar]
- 30.Yang ZX, Guo SP. A preliminary study on the population dynamics of Rattus norvegicus [in Chinese]. Acta Phytophylacica Sinica 1996; 23: 61–65. [Google Scholar]
- 31.Holly J, Lianne S, Thomas L. Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology 2005; 16: 717–726. [DOI] [PubMed] [Google Scholar]