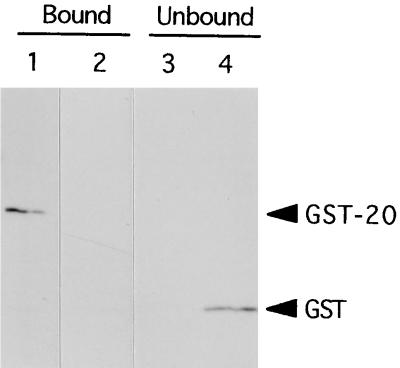
FIG. 6.
Coprecipitation of GST-20 with 45His. 45His was purified under denaturing conditions (100 mM CAPS [pH 10.5], 2 M urea, 20 mM imidazole containing 100 μg of p-APMSF per ml). For refolding, 300 μl of a 50% slurry of Ni-resin (Qiagen) that bound 45His was added to 30 ml of the binding buffer (100 mM CAPS [pH 10.5], 20% glycerol, 500 mM NaCl, 10 mM β-mercaptoethanol, 20 mM imidazole containing 100 μg of p-APMSF per ml) and rotated overnight at 4°C. The purified GST-Cry4A fusion proteins were then added, and the mixture was rotated for another 2 h. After centrifugation, the resin was washed five times with the binding buffer, and the supernatant was concentrated with microcon30 centrifugal filter device (Amicon). The resulting resin (bound fraction) and the concentrated supernatant (unbound fraction) were analyzed by SDS-PAGE (14% polyacrylamide) followed by Western blotting with the anti-GST antibody (Pharmacia Biotech). (A) GST-20 coprecipitated with 45His, but GST did not. (B) GST was detected in the supernatant. Lanes: 1, GST-20 and 45His; 2, GST and 45His.