SUMMARY
In March 2012, there was an unusual increase of gastroenteritis cases in a district with 37 264 inhabitants in central Greece. It was estimated that more than 3600 people developed symptoms. A 1:1 case-control study showed that consumption of tap water was a risk factor for acquiring infection [odds ratio (OR) 2·18, 95% confidence interval (CI) 1·11–4·28]. Descriptive data, low gastroenteritis incidence in adjacent areas with different water supply systems, and water-quality data further supported the hypothesis of a waterborne outbreak. Thirty-eight stool samples were positive for rotavirus. Bacterial indicators of recent faecal contamination were detected in samples from the water source and ice cubes from a local production enterprise. Molecular epidemiology of rotavirus strains, apart from the common strain, G3[P8], identified the unusual G/P combination G2P[8]. Water sanitation measures contributed to the control of the outbreak. This outbreak demonstrated the need for the cooperation of laboratories with different expertise and the importance of early notification of waterborne gastroenteritis outbreaks.
Key words: Gastroenteritis, outbreaks, rotavirus, virus infection, waterborne infections
INTRODUCTION
Waterborne gastroenteritis outbreaks (WBOs) caused by the consumption of contaminated drinking water are usually extensive since a large number of people are served by the same water supply system [1–3]. Several factors, such as the improvement of recording and the enhancement of laboratory methods have contributed to the recent increased detection of WBOs [4–5]. According to the data of the European Centre for Disease Control and Prevention, the actual incidence of WBOs is probably underestimated [6].
In Greece, notification of WBOs is included in the mandatory notification system. Thirteen WBOs were reported from 2004 to 2011 with a mean number of cases of 174 (s.d. = 231·4) [7–9]. Several bacteria, parasites and viruses have been identified as aetiological agents of WBOs worldwide, but very frequently the reported WBOs are of mixed aetiology [10–14].
On 14 March 2012, the Hellenic Center for Disease Control and Prevention (HCDCP) was informed by the Health Care Centre (HCC) of Elassona about an unusual increase of gastroenteritis cases since 10 March. Most cases were residents of Elassona, a district in central Greece with 37 264 inhabitants.
The city of Elassona (7233 inhabitants) and 5/52 villages of the district (1337 inhabitants) are served by the same water supply system, while the rest of the villages utilize autonomous systems (Fig. 1). The altitude of Elassona is 311 m and its geography includes farmlands in the valley areas, mountains to the west and east and forests in the west and east as well as grasslands. A river flows through Elassona and divides the town into two parts, which are connected by four bridges.
Fig. 1.
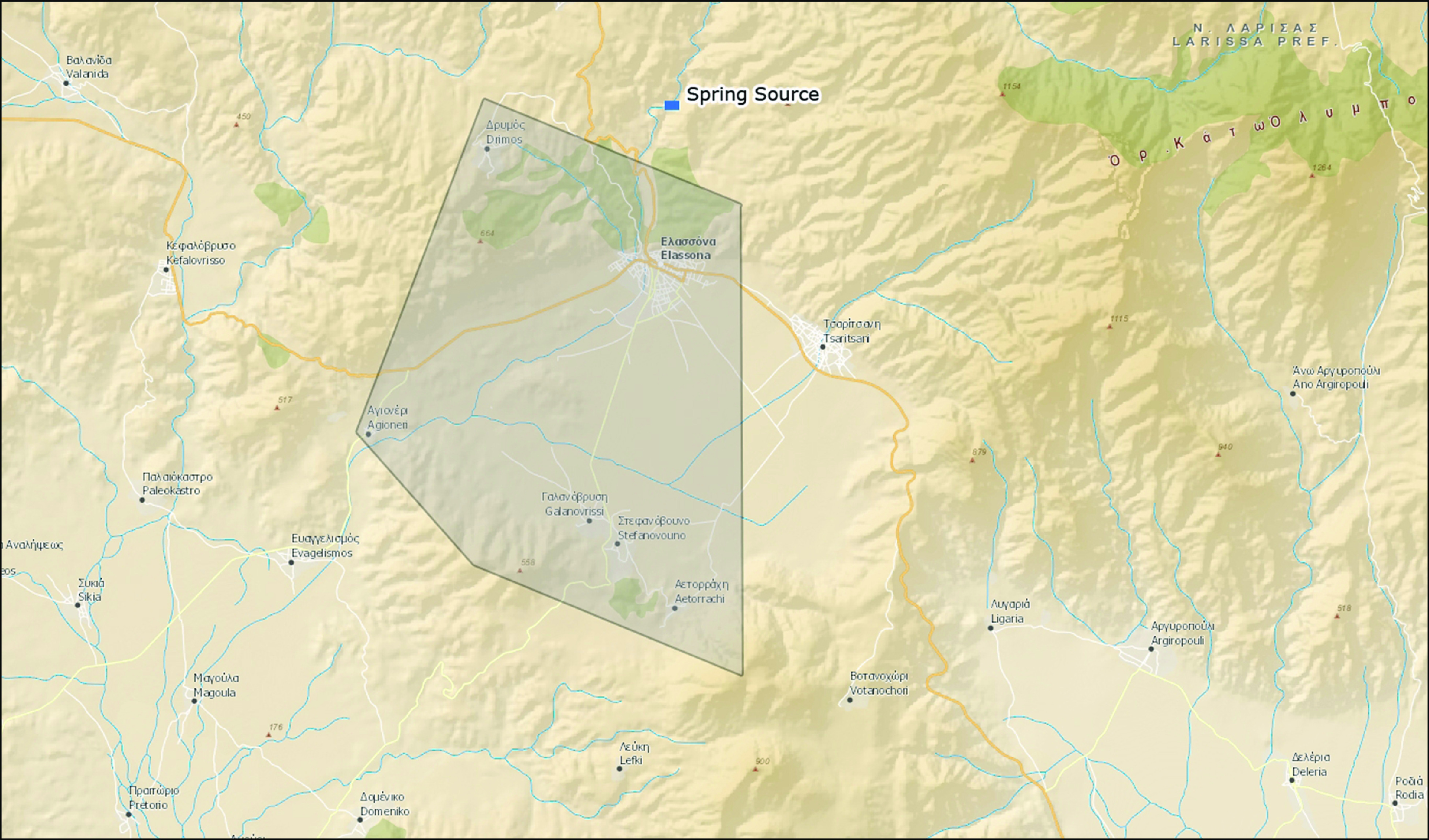
[colour online]. Map of Elassona district highlighting the area of Elassona city and the surrounding villages with the same water supply system, Greece, March 2012.
The main hypothesis formulated was that this was a WBO as there was heavy rainfall in the region the week before the onset of the gastroenteritis cases and several patients had reported that the water during the same period was coloured. In addition, cases were distributed in both parts of the town and similar outbreaks had occurred in the past during spring after heavy rains.
The HCDCP decided to further investigate the outbreak in collaboration with the local public health authorities. The objectives of the investigation were the identification of the mode and vehicle of transmission, as well as of possible risk factors, in order to implement appropriate control measures.
METHODS
Descriptive epidemiology and estimation of the extent of the outbreak
An active case-finding was initiated and medical doctors of Elassona HCC were requested to provide the total number of gastroenteritis cases from Elassona district they had treated since the 1 March and their demographic characteristics. Information on the place of residence and the date of symptom onset was also collected. To assess the extent of the outbreak more than 200 houses, distributed throughout the region, were visited. The total number of people living in each house and the number of persons that had developed gastroenteritis were recorded and the attack rate was estimated.
Finally, information on absence from schools (elementary and high schools) of Elassona and Tsaritsani, an adjacent city with 2507 residents that has a different water supply system, was collected. It should be noted that by the time the outbreak was notified, pre-schools/nurseries had already been closed because of the increased number of ill children. This is actually indicative of the high attack rate in toddlers.
Environmental investigation and water sampling
The spring water source of the supply system of Elassona is situated in a valley, 7 km north of the city, crossed by a river. The water of the river is in contact with the walls of the cement reservoir tank in which water from the source is gathered. In the basin of the river, several animal breeding farms (goats, sheep, cows, pigs) and houses are located. The spring water is transferred from the reservoir tank to a tank in the city, where chlorination takes place before distribution. Self-assessment microbiological tests are conducted by the municipality water authority on a monthly basis according to national legislation of water intended for human consumption (KYA Y2/38295/2007 ΦEK 630/26-4-2007). Almost half of the houses in Elassona are served by the public sewage system. Cases originated from houses with public or private systems.
The public water supply system was inspected on 16 of March by the Regional Public Health Laboratory (RPHL) of Thessaly.
On 16 March, health inspectors of RPHL of Thessaly collected six water samples – three for microbiological analysis and three for chemical analysis – from the source, the tank and tap water and placed a filter at the source (Gelman, Germany), for Cryptosporidium detection. Water samples were also collected on 6 April (n = 4) and 11 April (n = 4) after heavy rainfall, both from the tank and the source for microbiological and chemical analysis.
All water samples were obtained under sterile conditions, placed in sterile containers and transported to the laboratory in a cool box (5±3°C) within a few hours. The microbiological and chemical examination started immediately after the arrival of the samples at the laboratory. The samples were also sent to the Parasitology Department of the National School of Public Health (NSPH) and tested for Cryptosporidium by staining and polymerase chain reaction (PCR).
By 14 March, one water sample from the spring source and one from the tank had already been sent to the Environmental Microbiology Unit (EMU) of the University of Patras where molecular testing for rotavirus, adenovirus and norovirus was performed.
Analytical epidemiology
A case-control study with 1:2 ratio was designed. Since most of the cases were residents of Elassona, it was decided that the study population should be restricted to the residents of the city.
A probable gastroenteritis case was defined as any resident of Elassona that presented: (a) acute diarrhoea (three or more episodes) or (b) vomiting and one or more episodes of loose stools in a 24-h period between 6 and 17 March and a confirmed case as a probable case that was laboratory confirmed.
One hundred and fifty-one cases were randomly selected from the total recorded cases of the HCC for which the place of residence was known. Controls were selected from the residents of the city who had not developed gastroenteritis symptoms during the same period and were matched to cases by age group (<15, 15–30, 31–45, 46–60, >60 years) and gender (frequency matching). Controls were selected from the houses closest to the cases' houses. Each family provided no more than one case and one control.
A standardized questionnaire was used to obtain information on demographic characteristics, clinical manifestations and severity of the disease, prior contact with ill persons, participation in social activities/events, food consumption, water consumption and the number of glasses of water consumed daily. The questionnaire also included questions on a number of proxies for exposure to tap water: consumption of bottled water, use of tap water for washing fruit and vegetables, for cleaning teeth, for making ice cubes, and diluting juices, use of water filters, and use of a dish washer. All questions concerned the 10 days prior to the disease onset. Since the aetiological agent was unknown, the chosen time period was wide enough to cover a large spectrum of pathogens. Controls were asked about the same exposures in the 10-day period prior to 11 March, which was the day with the highest number of reported cases based on onset of symptoms. The questionnaires were completed door-to-door via person-to-person interviews. Along with the questionnaire, participants were asked to sign a consent form in which the purpose and conditions of the study were described. Mothers/guardians were asked to complete the questionnaire on behalf of children aged <15 years.
Laboratory investigation
Clinical samples
Initially, 27 stool samples were tested for bacteria (Salmonella spp., Shigella spp., Campylobacter spp., Yersinia and E. coli O157:H7), Cryptosporidium (Ziehl–Nielsen staining, immunofluorescence, PCR), norovirus and rotavirus (immunochromatography). A further 18 stool samples were tested for rotaviruses with rapid tests and PCR. Samples were collected at the HCC in sterile containers and transported to the laboratory in a cool box. They were immediately processed for nucleic acid extraction and rotavirus detection upon arrival. The detected rotaviruses were typed and the phylogenetic tree was constructed.
Water samples
Analysis for the classic bacterial indicators of spring water samples from the source and the tank was performed according to ISO methods. Samples were subjected to standard analysis for coliform bacteria and E. coli (ISO 9308-01: 2000), intestinal enterococci (ISO 7899-02: 2000) total count at 22°C, and at 37 °C (ISO 6222: 1999), and Clostridium perfringens (KYA 2600/2001). Water samples were also analysed for basic chemical parameters (pH, conductivity, nitrites, nitrates, chlorine residue). Finally, water samples were tested for Cryptosporidium/Giardia according to the EPA 1622 method and PCR, and for adenovirus, norovirus and rotavirus by real-time PCR.
Ice cube specimen collection and processing
A sample of ice cubes (each bag weighing 10 kg) produced from tap water 2 days after the water was reported as being coloured was collected from a local ice cube production enterprise. The sample was transported to the laboratory in a cool box and immediately underwent virological analysis for detection of human adenoviruses as an index virus of human faecal contamination and rotaviruses; bacteriological analysis was also performed according to ISO methods. Detection of virions was attempted by organic flocculation by the skimmed milk (SM) flocculation procedure as described previously [15]. The method is accredited in EMU of the University of Patras according to ISO 17025.
Nucleic acid extraction and enzymatic amplification
Viral nucleic acids were extracted from water and stool specimens using the QIAamp Viral RNA mini kit (Qiagen, Germany), utilizing the QIAcube fully automated platform. Real-time PCR and nested PCR techniques were used for the detection of adenoviruses, noroviruses, and rotaviruses, according to previously published protocols, by employing previously validated primer sets, targeting hexon, VP7/VP4, capsid and ORF1/2 for adenoviruses, rotaviruses, and noroviruses GI and GII, respectively [16–19]. Human adenovirus molecular detection was based on a TaqMan® assay described previously [20], in a MX3005P real-time PCR Platform (Stratagene, USA), using the TaqMan Universal PCR Master Mix (Applied Biosystems, USA). Extracted rotavirus dsRNA was first reverse-transcribed into gene 9 full-length cDNA with the generic primers Beg9/End9 [19]. Then, the cDNA product was used as a template for PCR VP7 amplification with the same Beg9/End9 pair of primers [17]. For P-typing, primers Con2-Con3 were used for amplification of a VP4-specific region [18]. The MJ Mini Personal Thermal Cycler (Bio-Rad, USA) was used.
Sequencing and phylogenetic analysis
Positive PCR products for rotavirus were purified using the QIAquick PCR purification kit (Qiagen, USA), and confirmed by sequencing (CeMIA, Greece) with Beg9 and Con2 primers for G- and P-typing, respectively. The obtained nucleotide sequences were analysed by the BLAST program at the NIH website (NCBI, National Centre for Technology Control, NIH, USA) and were compared with each other and with other published sequences. Multiple alignments were performed using the Molecular Evolutionary Genetics Analysis (MEGA) version 4.0.2 program. The neighbour-joining method was applied for phylogenetic tree analysis, the reliability of which was assessed by bootstrap resampling (1000 pseudo-replicates), using the MEGA.
Statistical analysis
Epi Info version 3.5.3 (CDC, USA) was used for data entry and SPSS v. 17.0 (SPSS Inc., USA) for data analysis. Contingency tables (univariate analysis) with the calculation of χ2 test or Fisher's exact test were used for the evaluation of the associations between the categorical variables. The odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were also calculated. Associations between categorical and quantitative variables were tested with Student's t test (for normally distributed variables), after checking for homoskedacity, or the Mann–Whitney test for skewed variables. P < 0·05 was considered statistically significant.
RESULTS
Descriptive epidemiology and estimation of the extent of the outbreak
Between 6 March and 1 April 2012, 552 cases were recorded at Elassona HCC. Of these, 341 were residents of the city of Elassona [attack rate (AR) 341/7233 = 4·7%], 53 were residents of adjacent villages with the same water supply system (AR: 53/1337 = 4·0%) and 158 were from villages with a different water supply system (AR 158/28 694 = 0·6%). The highest number of visits to the HCC was recorded on 11 March. Based on the temporal distribution of cases and the fact that chlorination took place on 15 March, cases that visited the HCC after 18 March (23·6% of the total cases) were considered as secondary cases of the outbreak, infected by person-to-person transmission. Gastroenteritis cases continued to attend the HCC until 1 April.
In the 200 houses visited by the researchers, an average of 50% of persons living in the same house had gastroenteritis symptoms. Thus, we can assume that the total number of cases in the city of Elassona, which is served by the same water supply system, was about 3620.
The percentages of absence from schools in Elassona and Tsaritsani at 12 March (1 day after the peak of the outbreak) were 37·6% (412/1097) and 20·2% (25/124), respectively. Under the assumption that the morbidity of children of both cities due to all other reasons was similar, the calculated relative risk of absence from Elassona's schools compared to Tsaritsani's schools was 1·86 (95% CI 1·30–2·67).
Results of the analytical study
Because of the high attack rate of the disease in the community, finding controls proved to be difficult, but eventually one control was finally obtained for each case. From the 151 cases randomly selected from the HCC records, 41 did not meet the probable case-definition criteria and nine had date of symptom onset after 18 March and were therefore considered as secondary cases of the outbreak.
In total, 101 cases and 151 controls were included in the case-control study. Age and gender distribution of cases and controls is presented in Table 1. Almost 40% of the cases were children (<15 years). Children aged <5 years accounted for 12% of all cases.
Table 1.
Distribution of cases (n = 101) and controls (n = 151) by gender and age group, Elassona, Greece, March 2012
| Cases | Controls | P value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender (male) | 45 | 44·6 | 73 | 48·3 | 0·555* |
| Age group (yr) | |||||
| <15 | 40 | 39·6 | 57 | 37·7 | 0·910 |
| 15–30 | 20 | 19·8 | 26 | 17·2 | |
| 31–45 | 8 | 7·9 | 15 | 9·9 | |
| 46–60 | 8 | 7·9 | 16 | 10·6 | |
| >60 | 25 | 24·8 | 37 | 24·5 | |
χ2 test.
The reported symptoms in cases were diarrhoea (100% as part of the case definition), fatigue (89·1%), loss of appetite (89·1%), abdominal pain (82·2%), vomiting (76·2%), arthralgia (54·2%) and fever (defined as body temperature >38°C) (45·5%). No cases of bloody diarrhoea were reported. Twenty (19·8%) cases visited the emergency room of the regional hospital but only two (2·0%) were admitted. The median reported duration of symptoms was 3 days (interquartile range 2–5 days).
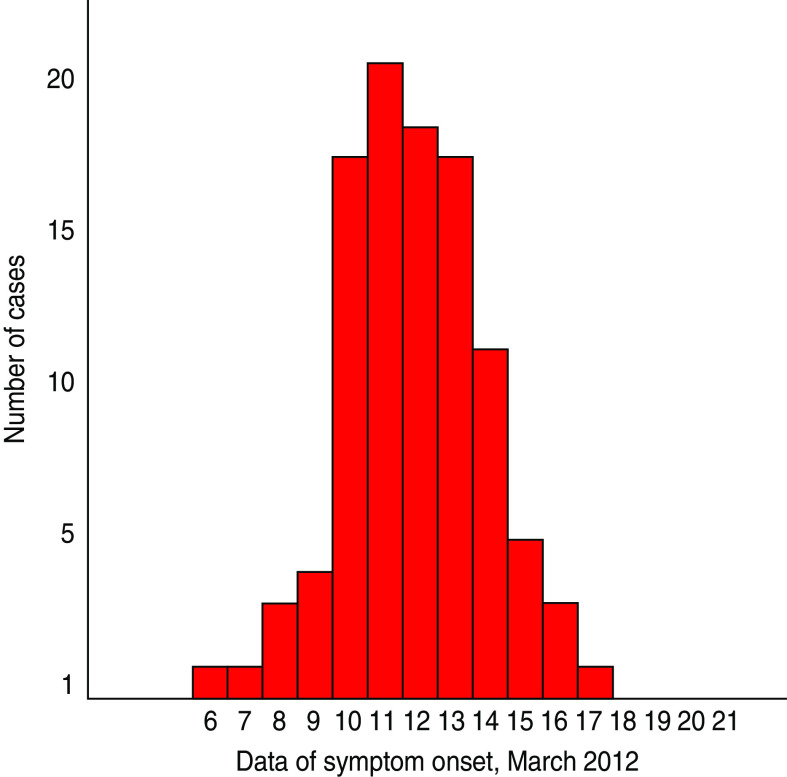
Figure 2 depicts the distribution of cases (n = 101) by date of symptom onset. The shape of the epidemic curve is compatible with a common point-source outbreak.
Fig. 2.
[colour online]. Distribution of cases (n = 101) by date of symptom onset, Elassona, Greece, March 2012.
According to the results of the univariate analysis (Table 2), the frequency of the disease was associated with the consumption of tap water (OR 2·18, 95% CI 1·11–4·28), while the number of daily glasses of tap water consumed did not significantly differ between cases and controls (P = 0·408). No food item was related to disease occurrence.
Table 2.
Results of univariate analysis, tap water consumption and tap water consumption proxies, Elassona, Greece, March 2012
| Exposure | Cases | Controls | OR | 95% CI | ||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||
| Consumption of tap water | 87/101 | 86·1 | 111/150 | 74·0 | 2·18 | 1·11–4·28 |
| Consumption of bottle water | 30/101 | 29·7 | 55/150 | 36·7 | 0·73 | 0·42–1·25 |
| Tap water for washing fruit and vegetables | 101/101 | 100·0 | 143/143 | 100 | n.a. | n.a. |
| Tap water for brushing teeth | 101/101 | 100·0 | 143/143 | 100 | n.a. | n.a. |
| Tap waterfor making ice cubes | 46/101 | 45·5 | 80/149 | 53·7 | 0·72 | 0·43–1·20 |
| Tap water for juice dilution | 68/101 | 67·3 | 95/142 | 66·9 | 1·02 | 0·59–1·76 |
| Use of dishwasher | 51/101 | 51·0 | 64/150 | 42·7 | 1·40 | 0·84–2·33 |
| Use of a water filter | 11/100 | 11·0 | 13/149 | 8·70 | 1·29 | 0·55–3·01 |
OR, Odds ratio, CI, confidence interval, n.a., not applicable.
Clinical samples
Testing of stool cultures for bacteria, parasites, and norovirus was negative. Rotaviruses were detected in 38/45 (84·4%) collected stool samples. The age of patients found to be shedding rotavirus was between 40 and 85 years.
Water samples
As shown in Table 3 the first spring water samples had an increased number of bacterial indicators of faecal contamination and there was no detectable free-chlorine residual. Under this context, water was characterized as inappropriate for consumption. In addition, testing of the water samples from the source and the tank for C. perfringens resulted in 238 c.f.u./100 ml and 223 c.f.u./100 ml, respectively. The examined chemical parameters were all within normal limits.
Table 3.
Summary of the results of laboratory testing of water samples, Elassona, Greece, March 2012
| Date of sampling | Sample source | Common aerobicmicrobes (c.f.u./ml) (36°C × 48 h) | Common aerobic microbes (c.f.u./ml) (22°C × 72 h) | Total coliforms (c.f.u./100 ml) | E. coli (c.f.u./100 ml) | Enterococci (c.f.u./100 ml) | Cl. perfringens (c.f.u./100 ml) | Detection of oocysts Cryptosporidium/Giardia (no.oocysts/100 l) |
|---|---|---|---|---|---|---|---|---|
| 14 Mar. 2012 | Spring source | 340 | 295 | 107 | 17 | 11 | 238 | — |
| 14 Mar. 2012 | Water tank | 15 | 20 | 105 | 5 | 0 | 223 | — |
| 16 Mar. 2012 | Spring source | 227 | 536 | 32 | 32 | 5 | 1 | — |
| 16 Mar. 2012 | Water tank | 24 | 27 | 0 | 0 | <4 | 0 | — |
| 16 Mar. 2012 | Filter | — | — | — | — | — | — | 0 |
| 16 Mar. 2012 | Tap water | 26 | 12 | 0 | 0 | 0 | — | — |
| 27 Mar. 2012 | Ice cubes | 174 | 600 | 15 | 8 | 31 | 4 | — |
| 06 Apr. 2012 | Spring source | 173 | 173 | 209 | 33 | 21 | 6 | — |
| 06 Apr. 2012 | Water tank | 23 | 30 | 0 | 0 | 0 | 0 | — |
| 11 Apr. 2012 | Water tank | 31 | 22 | 0 | 0 | 0 | 0 | — |
| 11 Apr. 2012 | Spring source | 74 | 218 | 14 | 6 | 12 | 0 | — |
Testing of ice cubes also resulted in an increased microbial load that rendered ice cubes inappropriate for consumption. Molecular testing of water samples and ice cubes was negative for rotavirus, adenovirus and norovirus.
Spring water samples collected on 6 and 11 April from the tank were free of bacteria, but samples from the source were found to be contaminated for both dates, although the measured microbial load was lower on the second occasion. The chemical examination was within normal values as well.
Molecular epidemiology of rotavirus strains
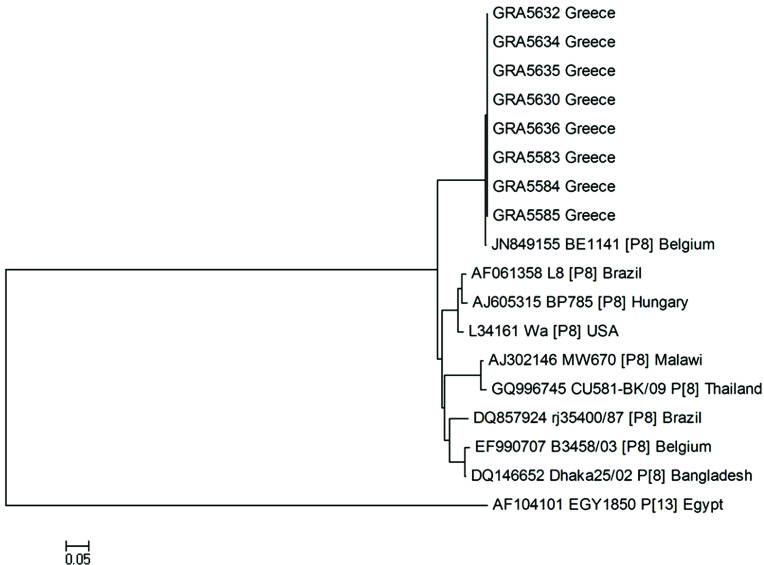
A phylogenetic tree was constructed for genomic segment 4. P[8] strains identified in the present study were closely related in the VP4 region to human rotavirus A strain BE1141 (GenBank: JN849155) detected in Belgium (99%) (Fig. 3).
Fig. 3.
Phylogenetic analysis of VP4 gene nucleotide sequences of the human rotavirus group A strains from the outbreak. A neighbour-joining phylogenetic tree was constructed using MEGA to represent phylogenetic relationships in 18 rotavirus strains. Eight P[8] strains from the outbreak and ten reference strains, the sequences of which were retrieved from the GenBank database, were included in the analysis. Two rotavirus strains of the study could not be P-typed. The bootstrap confidence levels were obtained for 1000 replicates. Sequence nomenclature shows the GenBank accession number, followed by strain designation, P type and country of origin. Rotavirus strains of this study are indicated by the prefix GR followed by sample ID. Rotavirus strain (GenBank: AF104101 P[13]) was used as an outgroup.
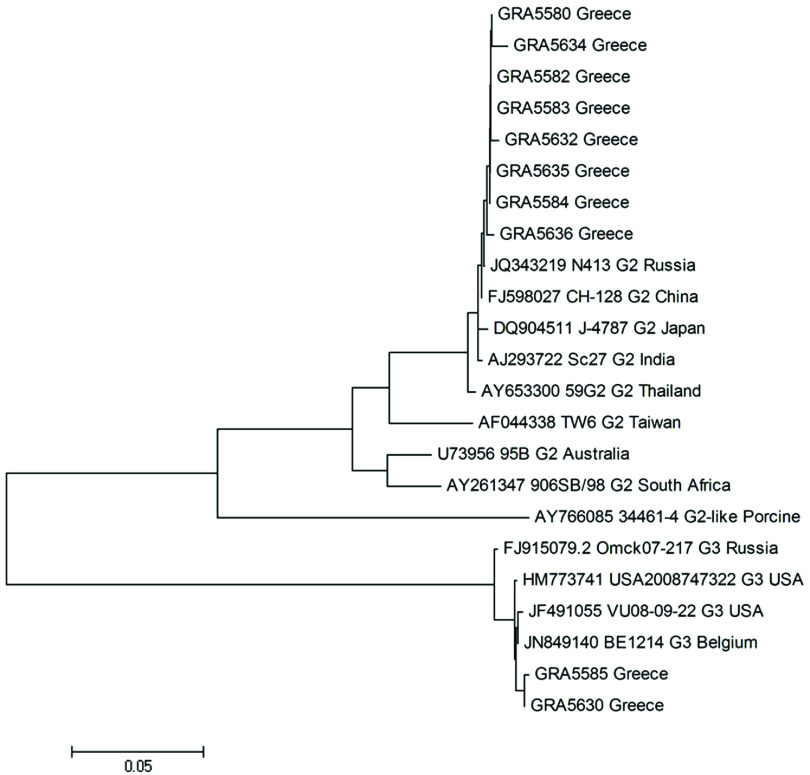
In the VP7 genome segment, sequences of G3 strains from the outbreak showed high similarities of 99% with strains JF491055 (USA), HM773741 (USA), and JN849140 (Belgium), with which they formed a cluster (Fig. 4). Finally, G2 strains of the present study, formed a cluster with strains JQ343219 (Russia), FJ598027 (China), DQ904511 (Japan), and AJ293722 (India), exhibiting high similarities of 99% (Fig. 4).
Fig. 4.
Phylogenetic analysis of VP7 gene nucleotide sequences of the human rotavirus group A strains from the outbreak. A neighbour-joining phylogenetic tree was constructed using MEGA to represent phylogenetic relationships in 23 rotavirus strains. Eight G2 and two G3 strains from the outbreak and 13 reference strains, the sequences of which were retrieved from the GenBank database, were included in the analysis. The bootstrap confidence levels were obtained for 1000 replicates. Sequence nomenclature shows the GenBank accession number, followed by strain designation, G type and country of origin. Rotavirus strains of this study are indicated by the prefix GR followed by sample ID.
Control measures
The occurrence of new cases gradually stopped after the concentration of residual chlorine in the water tank was increased to 1 ppm on 15 March for 24 h. For the prevention of similar future outbreaks the following measures were suggested:
-
(1)
Chlorination treatment and monitoring of chlorine concentration both in the water tank and at user points to be continued in compliance to the existing legislation (KYA Y2/38295/2007 ΦEK 630/26-04-2007).
-
(2)
In addition to the chlorination system, installation of a turbidimeter in the source for the measurement of water turbidity and in case of increased turbidity use of alternative water sources. According to European legislation (EU Drinking Water Directive 98/83/EC), the turbidity of the source of the water supply system should not exceed the value of 1·0 NTU (nephelometric turbidity unit).
-
(3)
Sampling of the water supply system at least twice a week in spring and after heavy rainfall in addition to the self-assessment quality samplings.
-
(4)
Systematic monitoring of the water supply system for 1 year to assess whether filtration of the water of the source is required.
DISCUSSION
In March 2012, a gastroenteritis outbreak that occurred in Elassona district, in central Greece, was investigated by the public health authorities with the support of several laboratories with different expertise. The investigation indicated that this was a large point-source outbreak with a peak on 11 March, with more than 3600 cases in the city of Elassona. Data also showed that it was followed by secondary person-to-person transmission after 18 March that further increased the disease burden in the community. One limitation to better describing the characteristics of the outbreak and accurately estimating the disease burden is that children were underestimated in the recorded cases at HCC. This is because parents tend to visit private paediatricians when their children become sick and when the disease is serious they prefer to directly visit the hospital instead of the local HCC. It should be noted that nurseries in Elassona had been closed almost from the beginning of the outbreak due to increased absenteeism, a fact that actually indicates the high attack rate of the disease in small children. Moreover, it should be emphasized that according to paediatricians about 30% of children aged <5 years were vaccinated against rotavirus. The vaccine offers protection against rotavirus gastroenteritis caused by G1 and non-G1 types (G3, G4, G9) when administered as a two-dose series in infants and children.
Descriptive epidemiological data (epidemic curve, geographical distribution of cases, etc.) and results of the analytical study supported a waterborne origin of the outbreak. The history of heavy rains at the beginning of the month and reports that the water was coloured, together with the water samples results, further support this hypothesis. In line with this was the observation of different rates of absence from schools in Elassona and an adjacent city with a different water supply system.
According to the revised strength-of-evidence classification of WBOs of the Centers for Diseases Control and Prevention (USA) [21], this outbreak could be considered as class I, since the available epidemiological data showed that the odds of having consumed water from the public water supply system before the disease occurrence was 2·2 times higher in gastroenteritis cases than in controls. In addition, there is also information supportive of a waterborne origin, i.e. no detectable free-chlorine residual and the presence of coliforms in the spring water samples that were tested.
Delayed detection, delayed implementation of water treatment measures and secondary person-to-person transmission of gastroenteritis contributed to the long duration of the outbreak (26 days). Delayed notification was the main limitation of the outbreak investigation. The aetiological agent could not be identified in the water samples since the first cases occurred around 6 March and the outbreak was not reported until 14 March when the water sampling took place. Delayed reporting of WBOs is a well known international problem for public health authorities, as well as underreporting [22]. The verification of the aetiological agent of WBOs is always a challenge as multi-pathogen outbreaks are common and adequate laboratory capacity for a variety of pathogens (bacteria, viruses, parasites, etc.) is required.
WBOs due to rotaviruses have been documented in the literature [23–25]; however, in most cases co-infection with a bacterium or another virus is identified. Surprisingly, 27 stool samples tested negative for norovirus, Salmonella spp., Shigella spp., Campylobacter spp., Yersinia, E. coli O157:H7, and Cryptosporidium. In addition, testing of water samples and ice cubes did not provide support for the hypothesis of the presence of another pathogen.
Presently, at least 27 G-types and 35 P-types are considered among rotavirus strains [26]. Studies have shown that five G types (G1–G4, G9) and two P types (P[4], P[8]) represent more than 90% of strains of clinical importance, globally [27]. In the context of this outbreak, G3P[8] and G2P[8] strains were identified. G3P[8] strains are among the most common human rotavirus genotypes circulating in Europe, while G2P[8] is an unusual G/P combination that in recent years has been detected in different parts of the world [27, 28], and is likely to be the consequence of a reassortment event between rotavirus strains with a Wa-like and a DS-1-like constellation, respectively [17, 26, 27]. All VP4 sequences from the Greek strains identified in the present study were identical (Fig. 3), which supports the view of a single P[8] shared by both G2 and G3 rotavirus strains. The possibility of a mixed infection, with an incomplete detection of all G and P genotypes involved, should not be excluded. Mixed infections are common and most likely represent naturally occurring reassortment in rotavirus strains [17, 27].
This outbreak investigation did not lead to a definitive conclusion on how the water of the source was contaminated and whether the faecal contamination was of human or animal origin. The extent of the outbreak and the observation that rotavirus strains were present in patients is suggestive that water was contaminated with human faeces from sewage, rather than from a single person. According to reports of large WBO investigations, most occur after the environmental contamination of the source combined with the failure of chlorination systems and the inadequate maintenance of the system [1, 25]. Contamination of the water source has been attributed to the agricultural run-off of sewage from dairy, sheep and cattle farms and nearby buildings in several studies [1, 22, 29, 30]. Heavy rains contribute to run-off of water from the fields into the rivers through mobilization of pathogens in the environment [31, 32] and usually lead to increased water turbidity [33]. The main hypothesis formulated in the context of this outbreak that heavy rains at the beginning of the month had resulted in such contamination of the source with human or animal waste from nearby houses and breeding farms, warrants further investigation.
This study shows that the early notification of WBOs is a prerequisite for appropriate investigation and identification of the aetiological agent in the water source. Furthermore, since: (a) the laboratory investigation of WBOs is complex, (b) their aetiology is usually mixed, and (c) in small countries like Greece no laboratory can have sufficient expertise for the full spectrum of pathogens that may be implicated, good collaboration between different laboratories and authorities should be established for investigation of WBOs.
ACKNOWLEDGEMENTS
The authors thank Georgia Papalagara and Miltiadis Tasoulis from the local public health authority, the municipality authorities of Elassona, Evaggelia Alexi, Athanasia Liapi and Antigoni Germanou from the HCCs and the personnel of the regional public health laboratory, Vasilis Nakoulas, Foteini Kolokythopoulou and Aggeliki Daskalaki.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Gallay A, et al. A large multi-pathogen waterborne community outbreak linked to faecal contamination of a groundwater system, France, 2000. Clinical Microbiology and Infection 2006; 12: 561–570. [DOI] [PubMed] [Google Scholar]
- 2.Crawn MF, et al. Waterborne outbreaks reported in the United States. Journal of Water and Health 2006; 4 (Suppl. 2): 19–30. [DOI] [PubMed] [Google Scholar]
- 3.Robertson LJ, et al. Cryptosporidium parvum infections in Bergen, Norway, during an extensive outbreak of waterborne giardiasis in autumn and winter 2004. Applied and Environmental Microbiology 2006; 72: 2218–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metcalf TG, Melnick JL, Estes MK. Environmental virology: from detection of virus in sewage and water by isolation to identification by molecular biology – a trip of over 50 years. Annual Review of Microbiology 1995; 49: 461–487. [DOI] [PubMed] [Google Scholar]
- 5.Grabow WOK, Taylor MB, de Villiers JC. New methods for the detection of viruses: call for review of drinking water quality guidelines. Water Science and Technology 2001; 43: 1–8. [PubMed] [Google Scholar]
- 6.European Centre for Disease Control and Prevention (http://www.ecdc.europa.eu/en/healthtopics/climate_change/health_effects/pages/water_borne_diseases.aspx). Accessed September 2012.
- 7.Parasidis T, et al. Outbreak of gastroenteritis occurred in North-Eastern Greece associated with several waterborne strains of noroviruses. International Journal of Infectious Diseases 2008; 12: E104–E105. [Google Scholar]
- 8.Karagiannis I, et al. A waterborne Campylobacter jejuni outbreak on a Greek island. Epidemiology and Infection 2010; 138: 1726–1734. [DOI] [PubMed] [Google Scholar]
- 9.Mellou K, et al. Waterborne infections and water-related gastroenteritis outbreaks. HCDCP Newsletter July 2011 (http://www2.keelpno.gr/blog/?page_id=30&lang=en). Accessed September 2012.
- 10.Leclerc H, Schwartzbrod L, Dei-Cas E. Microbial agents associated with waterborne diseases. Critical Reviews in Microbiology 2002; 28: 371–409. [DOI] [PubMed] [Google Scholar]
- 11.Daly ER, et al. Outbreak of giardiasis associated with a community drinking-water source. Epidemiology and Infection 2010; 138: 491–500. [DOI] [PubMed] [Google Scholar]
- 12.Kopilović B, et al. Waterborne outbreak of acute gastroenteritis in a costal area in Slovenia in June and July 2008. Eurosurveillance 2008; 13. [DOI] [PubMed] [Google Scholar]
- 13.O'Reilly CE, et al. A waterborne outbreak of gastroenteritis with multiple etiologies among resort island visitors and residents: Ohio, 2004. Clinical Infectious Diseases 2007; 44: 506–512. [DOI] [PubMed] [Google Scholar]
- 14.Baldursson S, Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks – an update 2004–2010. Water Research 2011; 45: 6603–6614. [DOI] [PubMed] [Google Scholar]
- 15.Calgua B, et al. Development and application of a one-step low cost procedure to concentrate viruses from seawater samples. Journal of Virological Methods 2008; 153: 79–83. [DOI] [PubMed] [Google Scholar]
- 16.Kokkinos P, et al. Molecular typing of enteroviruses, adenoviruses, and hepatitis A viruses in untreated and treated sewage of a biological treatment plant in Greece. Food and Environmental Virology 2010; 2: 89–96. [Google Scholar]
- 17.Barril PA, et al. Correlation between rotavirus A genotypes detected in hospitalized children and sewage samples in 2006, Córdoba, Argentina. Journal of Medical Virology 2010; 82: 1277–1281. [DOI] [PubMed] [Google Scholar]
- 18.Gentsch JR, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. Journal of Clinical Microbiology 1992; 30: 1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gouvea V, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. Journal of Clinical Microbiology 1990; 28: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernroth BE, et al. Environmental factors influencing human viral pathogens and their potential indicator organisms in the blue mussel, Mytilus edulis: the first Scandinavian report. Applied and Environmental Microbiology 2002; 68: 4523–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoder JS, et al. Surveillance for waterborne disease and outbreaks associated with recreational water use and other aquatic facility-associated health events – United States, 2005–2006. MMWR Surveillance Summaries 2008; 57 (SS09): 1–29. [PubMed] [Google Scholar]
- 22.Beaudeau P, et al. Lessons learned from ten investigations of waterborne gastroenteritis outbreaks, France, 1998–2006. Journal of Water and Health 2008; 6: 491–503. [DOI] [PubMed] [Google Scholar]
- 23.Corwin AL, et al. A large outbreak of probable rotavirus in Nusa Tenggara Timur, Indonesia. American Journal of Tropical Medicine and Hygiene 2005; 72: 488–494. [PubMed] [Google Scholar]
- 24.Divizia M, et al. Waterborne gastroenteritis outbreak in Albania. Water Science and Technology 2004; 50: 57–61. [PubMed] [Google Scholar]
- 25.Koroglu M, et al. A waterborne outbreak of epidemic diarrhoea due to group A rotavirus in Malatya, Turkey. New Microbiologica 2011; 34: 17–24. [PubMed] [Google Scholar]
- 26.Matthijnssens J, et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Archives of Virology 2011; 156: 1397–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Reviews in Medical Virology 2005; 15: 29–56. [DOI] [PubMed] [Google Scholar]
- 28.De Donno A, et al. Emergence of unusual human rotavirus strains in Salento, Italy, during 2006–2007. BMC Infectious Diseases 2009; 9: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanley K, Cunningham R, Jones K. Thermophilic campylobacters in groundwater. Journal of Applied Microbiology 1998; 85: 187–191. [DOI] [PubMed] [Google Scholar]
- 30.Le Guyader F, et al. Three-year study to assess human enteric viruses in shellfish. Applied and Environmental Microbiology 2000; 66: 3241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunter PR. Climate change and water-borne and vector-borne disease. Journal of Applied Microbiology 2003; 94 (Suppl): 37S–46S. [DOI] [PubMed] [Google Scholar]
- 32.Cann KF, et al. Extreme water-related weather events and waterborne disease. Epidemiology and Infection 2012; 9: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tinker SC, et al. Drinking water turbidity and emergency department visits for gastrointestinal illness in Atlanta, 1993–2004. Journal of Exposure Science and Environmental Epidemiology 2010; 20: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]