Abstract
The vaccine for the coronavirus disease 2019 (COVID‐19) has been reported to potentially cause or worsen diabetes. A 73‐year‐old Japanese woman received two doses of Moderna COVID‐19 vaccine. Four weeks after the second vaccination, her glycemic control began to deteriorate, and 8 weeks after the second vaccination, the patient was diagnosed with new‐onset type 1 diabetes that was strongly positive for autoantibodies and showed a disease‐susceptible human leukocyte antigen haplotype, DRB1*04:05:01‐DQB1*04:01:01. The glucagon stimulation test suggested an insulin‐dependent state, and induction of intensive insulin therapy brought about fair glycemic control. The time period from the COVID‐19 vaccination to the development of type 1 diabetes was relatively longer than to the onset or exacerbation of type 2 diabetes, as previously reported, suggesting the complicated immunological mechanisms for the destruction of β‐cells associated with the vaccination. In recipients with the disease‐susceptible haplotypes, one should be cautious about autoimmune responses for several months after the vaccination.
Keywords: COVID‐19, Type 1 diabetes, Vaccine
We experienced a case of newly diagnosed type 1 diabetes with strongly positivity for autoantibodies that developed after the coronavirus disease 2019 vaccination and showed a disease‐susceptible human leukocyte antigen haplotype. The time period from the coronavirus disease 2019 vaccination to the development of type 1 diabetes is relatively longer than to the onset or exacerbation of type 2 diabetes, as previously reported, suggesting the complicated immunological mechanisms for the destruction of β‐cells associated with the vaccination. In recipients with the disease‐susceptible haplotypes, one should be cautious about autoimmune responses for several months after the vaccination.

INTRODUCTION
Since December 2019, the world has been suffering from a severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) outbreak. The coronavirus disease 2019 (COVID‐19) triggers an inflammatory immune response called a cytokine storm, which affects not only respiratory systems, but also the digestive, circulatory, renal and central nervous systems, leading to multiple organ failure 1 . The relationship between COVID‐19 and diabetes has been reported in terms of serious complications, such as diabetic ketoacidosis and new‐onset diabetes, which are mainly due to the impaired β‐cell functions 2 . Recent reports have shown that direct infection with SARS‐CoV‐2 on β‐cells induces β‐cell apoptosis 3 and transdifferentiation 4 . Decreased β‐cell mass is a common pathology in both type 1 and type 2 diabetes, and it has been suggested that SARS‐CoV‐2 infection might worsen glycemic control by simultaneously causing β‐cell apoptosis and transdifferentiation, resulting in β‐cell loss 2 . COVID‐19 vaccine was developed to prevent an ongoing pandemic, and has been administered with high efficacy and safety. However, as administration of COVID‐19 vaccine has progressed, the possibility of worsening glycemic control after the vaccination has also been reported 5 , 6 , 7 , 8 . We experienced a case of newly diagnosed type 1 diabetes strongly positive for autoantibodies that developed after the vaccination and showed a disease‐susceptible human leukocyte antigen haplotype.
CASE REPORT
A 73‐year‐old Japanese woman, who had a history of osteoporosis and non‐tuberculous mycobacterial infection, had mild glucose intolerance and had been treated with diet and exercise without antidiabetic medications for 7 years. Three months before the first COVID‐19 vaccination, her glycated hemoglobin (HbA1c) level was >7%, meeting the American Diabetes Association's diagnostic criteria for diabetes. Subsequently, her HbA1c level decreased without further intervention. None of the patient’s relatives had diabetes or autoimmune diseases. The patient received two doses of Moderna COVID‐19 vaccine (Spikevax®, mRNA‐1273; Cambridge, MA, USA) at a 4‐week interval. The day after both vaccinations, the patient had a slight fever and malaise, but these symptoms resolved within a few days. However, 4 weeks after the second vaccination, despite no change in her lifestyle, her HbA1c level rose to 7.3%, and 3 weeks later, anorexia, fatigue, nausea and vomiting were observed.
Eight weeks after the second vaccination, although her vital signs were stable and a test of urinary ketone was negative, blood tests showed a HbA1c level of 9.3%, casual blood glucose level of 318 mg/dL, deteriorating blood glucose control, serum C‐peptide level of 1.80 ng/mL, anti‐glutamic acid decarboxylase antibody level of >2,000 U/mL and insulin autoantibody level of 581 NU/mL, suggesting the development of type 1 diabetes. Other relevant laboratory results are summarized in Table 1. The polymerase chain reaction test was negative for COVID‐19 and there were no findings of infection, including exacerbation of non‐tuberculous mycobacterial infection. Dynamic computed tomography scanning showed no malignancies in the liver or pancreas that could contribute to worsening glycemic control. The glucagon stimulation test carried out 11 days after the diagnosis of type 1 diabetes suggested an insulin‐dependent state; the difference in serum C‐peptide level before and after stimulation was 0.48 ng/mL (serum C‐peptide level before and 6 min after glucagon stimulation was 0.54 and 1.02 ng/mL, respectively). Human leukocyte antigen deoxyribonucleic acid typing identified that the patient was homozygous for one of the susceptible human leukocyte antigen haplotypes for type 1 diabetes, DRB1*04:05:01‐DQB1*04:01:01.
Table 1.
Blood and urinary data of the patient
| Result | Reference range | Result | Reference range | ||
|---|---|---|---|---|---|
| Blood biochemistry | Peripheral blood | ||||
| TP (g/dL) | 7.2 | 6.6–8.1 | WBC (/mm3) | 6,600 | 3,300–8,600 |
| Alb (g/dL) | 4.3 | 4.1–5.1 | RBC (×104/mm3) | 429 | 386–492 |
| T Bil (mg/dL) | 1.2 | 0.4–1.5 | Hb (g/dL) | 13.1 | 11.6–14.8 |
| BUN (mg/dL) | 31.4 | 8–20 | Ht (%) | 39.4 | 35.1–44.4 |
| Cr (mg/dL) | 0.88 | 0.46–0.79 | Plt (×104/mm3) | 22.4 | 15.8–34.8 |
| UA (mg/dL) | 4.0 | 2.6–7.0 | |||
| Na (mEq/L) | 136.2 | 138–145 | Immunological data | ||
| K (mEq/L) | 4.1 | 3.6–4.8 | GADAb (U/mL) | ≥2,000 | 0–4.9 |
| Cl (mEq/L) | 98 | 101–108 | IAA (NU/mL) | 581 | 0–124 |
| Glucose (mg/dL) | 318 | 73–109 | IA‐2Ab (U/mL) | <0.6 | 0–0.6 |
| HbA1c (%) | 9.3 | 4.9–5.9 | ICA | Negative | Negative |
| Glycoalbumin (%) | 34.9 | 11.7–15.8 | ZnT8Ab (U/mL) | <10.0 | <15.0 |
| CPR (ng/mL) | 1.80 | 0.74–3.18 | TRAb (IU/L) | <0.9 | 0–1.9 |
| TG (mg/dL) | 130 | 30–149 | TgAb (IU/mL) | <5.0 | 0–5.0 |
| HDL‐C (mg/dL) | 70 | 40–103 | TPOAb (IU/mL) | <3.0 | 0–3.0 |
| LDL‐C (mg/dL) | 166 | 65–139 | |||
| AST (U/L) | 20 | 13–30 | HLA allele | DRB1*04:05:01 | Homozygous |
| ALT (U/L) | 29 | 7–23 | DQB1*04:01:01 | Homozygous | |
| LDH (U/L) | 175 | 124–222 | DQA1*03:03 | Homozygous | |
| ALP (U/L) | 62 | 38–113 | |||
| ɤ‐GTP (U/L) | 97 | 9–32 | Urinary test | ||
| AMY (U/L) | 80 | 44–132 | Urinary glucose | 3+ | – |
| CK (U/L) | 94 | 41–153 | Urinary protein | – | – |
| CRP (mg/dL) | 0.04 | 0–0.14 | Urinary blood | 1+ | – |
| TSH (µIU/mL) | 1.650 | 0.61–4.23 | Urinary ketone body | – | – |
| fT3 (pg/mL) | 2.8 | 2.0–4.5 | Urinary bilirubin | – | – |
| fT4 (ng/dL) | 1.6 | 0.7–1.8 | Microalbumin (mg/gCr) | 8.4 | 0–29.9 |
Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMY, amylase; AST, aspartate transaminase; BUN, blood urea nitrogen; CK, creatine kinase; Cl, chlorine; CPR, connecting peptide immunoreactivity; Cr, creatinine; CRP, C‐reactive protein; fT3, free triiodothyronine; fT4, free thyroxine; GADAb, glutamic acid decarboxylase antibody; ɤ‐GTP, gamma‐glutamyl transferase; Hb, hemoglobin; HbA1c, glycated hemoglobin A1c; HDL‐C, high density lipoprotein cholesterol; HLA, human leukocyte antigen; Ht, hematocrit; IA‐2Ab, insulinoma‐associated protein‐2 antibody; IAA, insulin autoantibody; ICA, islet cell antibody; K, potassium; LDH, lactate dehydrogenase; LDL‐C, low density lipoprotein cholesterol; Na, sodium; Plt, platelet; RBC, red blood cell; T Bil, total bilirubin; TG, triglyceride; TgAb, thyroglobulin antibody; TP, total protein; TPOAb, thyroid peroxidase antibody; TRAb, thyroid‐stimulating hormone receptor antibody; TSH, thyroid‐stimulating hormone; UA, uric acid; WBC, white blood cell; ZnT8Ab, zinc transporter 8 autoantibody.
Induction of intensive insulin therapy achieved and maintained fair glycemic control, and all the patient's symptoms associated with hyperglycemia disappeared. The patient had neither diabetic microvascular complications nor autoimmune thyroid diseases. The blood test carried out 12 weeks after the second vaccination showed that fasting blood glucose and serum C‐peptide levels were 89 mg/dL and 0.42 ng/mL respectively, showing that the patient was still insulin‐dependent. The change in HbA1c before and after the vaccination is shown in Figure 1.
Figure 1.
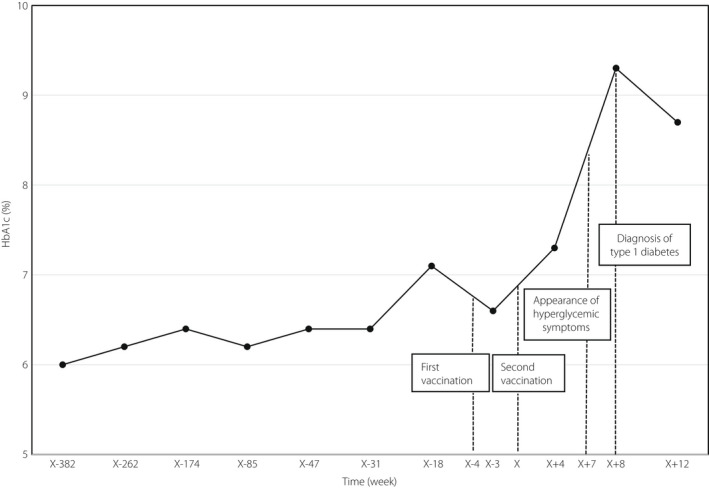
Glycated hemoglobin (HbA1c) changes before/after coronavirus disease 2019 vaccine administration. The reference date of the time axis (day X) was set as the date of the second vaccination.
Written informed consent was obtained from the patient. As the present study was not a clinical study, but a case report, no ethical approval was required.
DISCUSSION
Because patients diagnosed with COVID‐19 have been reported to develop diabetes more frequently and might be at increased risk for hyperglycemia, 9 vaccines that produce virus‐like proteins could similarly make recipients develop or exacerbate diabetes, and have recently been reported to worsen glycemic control. 5 , 6 , 7 , 8 Although most of the cases concluded to be the onset or exacerbation of type 2 diabetes occurred early after the first or second vaccination, 5 , 6 , 7 the diagnosis of type 1 diabetes took a relatively long period (4–8 weeks) after the second vaccination, including the previously reported 8 case as well as the present case. The difference in the duration between the vaccination and the onset of hyperglycemia could imply multiple and complicated mechanisms for the development of hyperglycemia associated with the vaccination. Although Edwards et al. 7 speculated that a direct insult to β‐cells by the pro‐inflammatory cytokines induced by SARS‐CoV‐2 is a common mechanism for worsening glycemic control caused by SARS‐CoV‐2 infection and vaccination, the accumulation of autoreactive immune cells to β‐cells might require a certain period, because of the process of cross‐immunity in which T cells presented with a portion of the viral antigen protein coded by inserted messenger ribonucleic acid (mRNA) through mRNA vaccination in antigen‐presenting cells acquire autoimmunity against β‐cells.
The administration of adjuvants to an individuals genetically predisposed to the disease can cause serious adverse effects through activation of autoimmune cascades and pathways, which is called autoimmune/inflammatory syndrome induced by adjuvants (ASIA syndrome). 8 , 10 Although Moderna COVID‐19 vaccine does not contain any additional adjuvants, polyethylene glycol lipid conjugates contained in the COVID‐19 mRNA vaccines might act as an adjuvant and induce autoimmune responses, and mRNA appears to have self‐adjuvant properties. 8 As the diagnostic criteria for ASIA syndrome were met in this case, 10 it might be considered as another potential mechanism for the development of type 1 diabetes associated with COVID‐19 vaccination.
The patient, who had no episodes of infection preceding the onset of the disease, and was under good glycemic control over a long period with good adherence to dietary and exercise therapy, suddenly showed hyperglycemic symptoms and a quite high HbA1c value with high titers for autoantibodies against glutamic acid decarboxylase and insulin, suggesting the development of type 1 diabetes attributable to COVID‐19 vaccination. Because patients with the disease‐susceptible haplotype, as in the present case, might need to be monitored for autoimmune responses for several months after the vaccination, accumulation and analysis of similar cases of diabetes associated with the vaccination and elucidation of the mechanism are warranted.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: N/A.
Informed consent: Written informed consent was obtained from the patient .
Approval date of registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
J Diabetes Investig. 2022; 13: 1105–1108
REFERENCES
- 1. Pollard CA, Morran MP, Nestor‐Kalinoski AL. The COVID‐19 pandemic: a global health crisis. Physiol Genomics 2020; 52: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shirakawa J. Pancreatic β‐cell fate in subjects with COVID‐19. J Diabetes Investig 2021; 12: 2126–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu C‐T, Lidsky PV, Xiao Y, et al. SARS‐CoV‐2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab 2021; 33: 1565–1576.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang X, Uhl S, Zhang T, et al. SARS‐CoV‐2 infection induces beta cell transdifferentiation. Cell Metab 2021; 33: 1577–1591.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abu‐Rumaileh MA, Gharaibeh AM, Gharaibeh NE. COVID‐19 vaccine and hyperosmolar hyperglycemic state. Cureus 2021; 13: e14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee HJ, Sajan A, Tomer Y. Hyperglycemic emergencies associated with COVID‐19 vaccination: a case series and discussion. J Endocr Soc 2021; 5: bvab141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edwards AE, Vathenen R, Henson SM, et al. Acute hyperglycaemic crisis after vaccination against COVID‐19: A case series. Diabetes Med 2021; 38: e14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patrizio A, Ferrari SM, Antonelli A, et al. A case of Graves' disease and type 1 diabetes mellitus following SARS‐CoV‐2 vaccination. J Autoimmun 2021; 125: 102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Metwally AA, Mehta P, Johnson BS, et al. COVID‐19‐induced new‐onset diabetes: trends and technologies. Diabetes 2021; 70: 2733–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bragazzi NL, Hejly A, Watad A, et al. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metab 2020; 34: 101412. [DOI] [PubMed] [Google Scholar]


