Fig. 2.
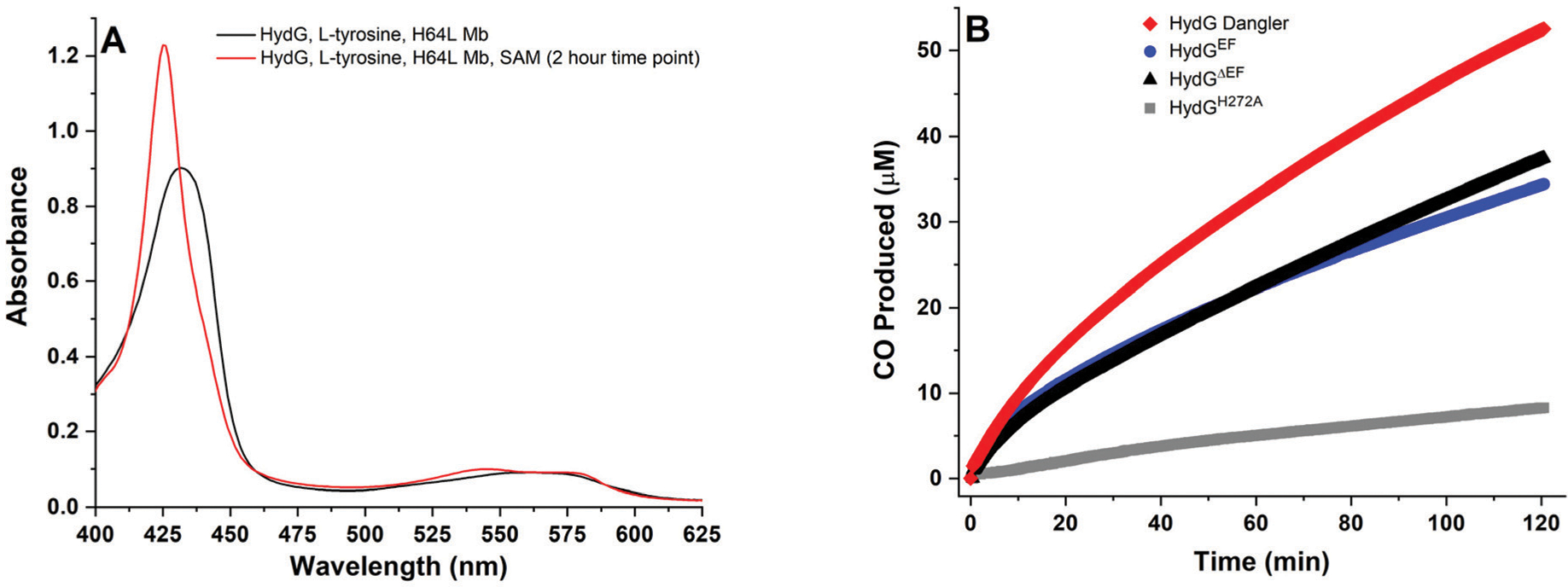
HydG catalyzed CO formation, as monitored via the spectroscopic changes associated with CO binding to deoxy H64L myoglobin at 37 °C. A. H64L myoglobin (80 μM heme) in the presence of 8 mM DT, 600 μM l-tyrosine, and WT dangler reconstituted HydGΔEF (10 μM protein with 8.23 ± 0.43 Fe per protein). Black spectrum, mixture before addition of 1 mM SAM; red spectrum, 2 hours after SAM was added to initiate catalysis. B. CO formed during turnover experiments as monitored via single wavelength kinetics at λ = 425 nm at 37 °C in 50 mM Tris, 10 mM KCl, pH 8.1 buffer. Traditionally reconstituted H272A HydGΔEF (gray, 25 μM protein with 7.65 ± 0.26 Fe per protein); WT traditionally reconstituted HydGΔEF (black, 10 μM protein with 7.54 ± 0.48 Fe per protein); WT traditionally reconstituted HydGEF (blue, 10 μM protein with 7.38 ± 0.40 Fe per protein); and WT dangler reconstituted HydGΔEF (red, 10 μM protein with 8.23 ± 0.43 Fe per protein).
