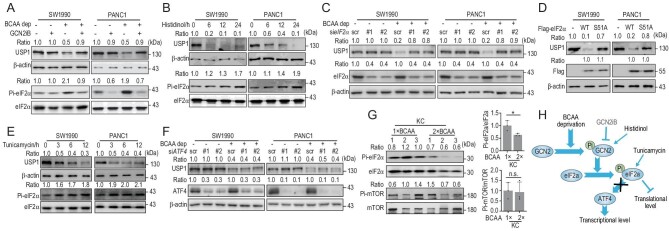
Figure 4.
BCAA increases USP1 protein via the GCN2-eIF2α pathway. (A) GCN2 inhibitor (GCN2iB, 5 μM) rescues the USP1 protein level with BCAA deprivation. ‘Ratio’, upper panel: USP1/β-actin; lower panel: Pi-eIF2α/eIF2α. (B) Histidinol (2 mM) decreases the USP1 protein level in a time-dependent manner. ‘Ratio’, upper panel: USP1/β-actin; lower panel: Pi-eIF2α/eIF2α. (C) Knockdown of eIF2α rescues the USP1 protein level under the BCAA deprivation condition. ‘Ratio’, upper panel: USP1/β-actin; lower panel: eIF2α/β-actin. (D) Overexpression of eIF2α wild-type but not enzyme-dead S51A mutant dramatically decreases the USP1 protein level. ‘Ratio’, upper panel: USP1/β-actin; lower panel: Flag/β-actin. (E) Tunicamycin (5 μg/mL) attenuates the USP1 protein level in a time-dependent manner. ‘Ratio’, upper panel: USP1/β-actin; lower panel: Pi-eIF2α/eIF2α. (F) ATF4 knockdown fails to rescue the USP1 protein level under the BCAA deprivation condition. ‘Ratio’, upper panel: USP1/β-actin; lower panel: ATF4/β-actin. (G) A higher BCAA diet decreases phosphorylation of eIF2α and has no effect on mTOR in KC mice. Mean ± SEM of n = 3 biologically independent experiments, two-tailed t-test. (H) Working model. BCAA deprivation activates the GCN2-eIF2α pathway and blocks USP1 protein synthesis. Data in (A–G) are representative of three biologically independent experiments. n.s. donates for no significance, *P < 0.05.