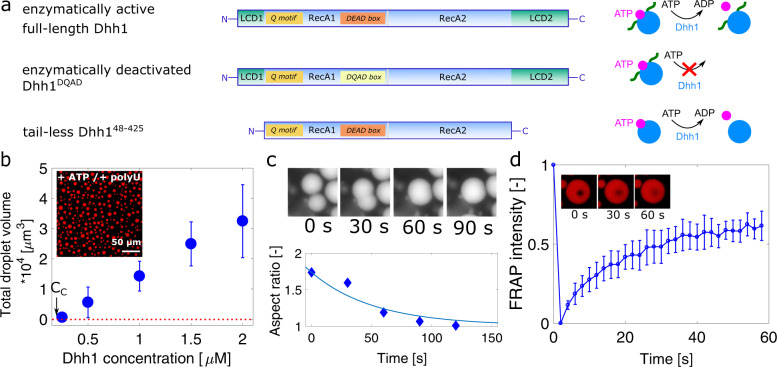
Fig. 1. In vitro phase separation of Dhh1.
a Dhh1 variants used in this study: full-length Dhh1, consisting of two RecA domains as core, flanked by an N- and a C-terminal low-complexity domain (LCD1/2). Q motif and DEAD box are important motifs to bind and hydrolyze ATP. In the Dhh1DQAD variant, a single glutamate has been substituted with a glutamine, leading to an ATP hydrolysis-deficient variant, while the tail-less Dhh148–425 construct lacks both the N- and C-terminal LCD. b Effect of Dhh1 concentration on droplet formation. Dhh1 solutions at increasing protein concentrations were supplied with 5 mM ATP/MgCl2 and 0.5 mg/ml polyU and imaged using widefield fluorescence microscopy providing a critical concentration (CC) equal to 0.2 µM. The panel shows the average total droplet volume of three different samples, with error bars representing the standard deviation. c Fusion and relaxation of two representative droplets into one single droplet, quantified by measuring the aspect ratio of two fusing droplets over time. d Mean FRAP signal of three different droplets of the droplets shown in (b), exhibiting recovery of 61 ± 2%. Error bars represent the standard deviation of the FRAP signal of three different droplets. Source data for panels b–d are provided in the Source Data file.