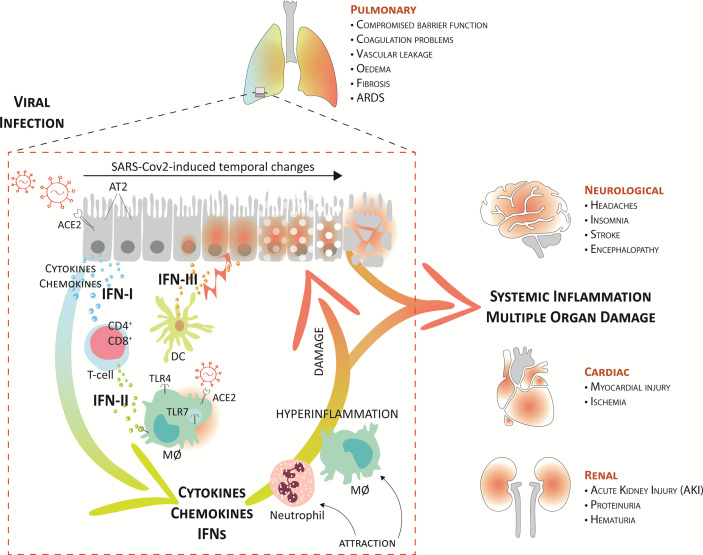
Figure 3.
Systemic inflammation and multiple organ damage in severe COVID-19. A current model suggests that upon upper airway entry, SARS-CoV-2 impregnates the lower lungs via ACE2, where it infects several target cells, including the alveolar type 2 cells (AT2), alveolar macrophages (MØ), and vascular endothelial cells. Virus cell entry recognition via endosomal and cytosolic pattern recognition receptors (as TLR3 and TLR7) trigger the production of IFNs and proinflammatory mediators. Together, the local recruitment of effector T-cells (CD4+ and CD8+) and dendritic cells (DCs), further perpetuates higher proinflammatory cytokines, IFNs, and chemokines in the lung fluid. Consequently, this further augments damage to the lung tissue, mediated by endothelial dysfunction and vasodilation, and leads to the attraction of more inflammatory neutrophils and macrophages that create a pathogenic inflammatory loop in the lung. On the one hand, this leads to respiratory/organ failure and on the other hand, it also results in a “systemic cytokine storm” that causes widespread inflammation and damage to other vulnerable organs, like the heart, kidney, and brain. This can rapidly progress to multiple organ exhaustion, which correlates with a poor prognosis in COVID-19 patients.