Summary
Background
Early 2-dose measles vaccine (MV) at 4 and 9 months of age vs. the WHO strategy of MV at 9 months of age reduced all-cause child mortality in a previous trial. We aimed to test two hypotheses: 1) a 2-dose strategy reduces child mortality between 4 and 60 months of age by 30%; 2) receiving early MV at 4 months in the presence versus absence of maternal measles antibodies (MatAb) reduces child mortality by 35%.
Methods
Single-centre open-label community-based randomised controlled trial in Guinea-Bissau, with 2:1 block-randomisation by sex to a 2-dose (4 + 9 months) vs. 1-dose (9 months) MV strategy. Healthy children were eligible 4 weeks after the 3rd diphtheria-tetanus-pertussis-containing vaccine. Before randomisation a blood sample was collected to determine MatAb level. The primary outcome was all-cause mortality. Hazard ratios (HR) were derived from Cox regression in the per protocol population. We tested for interactions with national campaigns with oral polio vaccine (C-OPV). Trial registration: NCT01486355.
Findings
Between August 2011-April 17th 2015, 6,636 children were enroled, 6,598[n2-dose=4,397; n1-dose=2,201] were included in the analysis of the primary outcome, The HR(2-dose/1-dose) between 4 and 60 months was 1.38 (95%CI: 0.92–2.06) [deaths: n2-dose=90; n1-dose=33]. Before the 9-month MV and the HR(1-dose/no dose) was 0.94 (0.45–1.96) [deaths: n2-dose=21; n1-dose=11]. The HR(2-dose/1-dose) was 0.81 (0.29–2.22) for children, who received no C-OPV [deaths/children: n2-dose=10/2,801; n1-dose=6/1,365], and 4.73 (1.44–15.6) for children, who received C-OPV before and after enrolment (p for interaction=0.027) [deaths/children: n2-dose=27/1,602; n1-dose=3/837]. In the 2-dose group receiving early MV at 4 months, mortality was 50% (20–68%) lower for those vaccinated in the presence of MatAb vs. the absence of MatAb [deaths/children: nMatAb=51/3,132; nnoMatAb=31/1,028].
Interpretation
The main result contrasts with previous findings but may, though based on a small number of events, be explained by frequent OPV campaigns that reduced the mortality rate, but apparently interacted negatively with early MV. The beneficial non-specific effects of MV in the presence of MatAb should be investigated further.
Funding
ERC, Danish National Research Foundation, the Danish Council for Development Research, Ministry of Foreign Affairs, Novo Nordisk Foundation, European Union and the Lundbeck Foundation.
Keywords: Measles, Mortality, Vaccines, Maternal antibody, Non-specific effects, Heterologous effecs, Maternal priming
Research in context.
Evidence before this study
We searched for other trials investigating maternal measles antibody (MatAb) and non-specific effects of vaccines on PubMed. We searched in August 2021 with the term: "maternal measles antibody non-specific effect". We identified one relevant paper. We additionally obtained papers known to and recommended by our co-authors on this paper.
The World Health Organisation (WHO) recommends delaying measles vaccination until MatAb has waned, because the presence of MatAb lowers the child's serological response to measles vaccine (MV). However, it may be a mistake: a previous randomised trial showed that an early 2-dose MV strategy was associated with a 30% (95%CI: 6–48%) reduction in all-cause mortality between 4 and 36 months; this effect was not explained by prevention of measles infection. Intriguingly, receiving early MV in the presence of MatAb was associated with lower all-cause mortality compared with receiving early MV in the absence of MatAb. Three cohort studies have subsequently confirmed the particular survival benefit of receiving MV in presence of MatAb.
Added value of this study
We designed the present study to test the hypotheses that 1) a 2-dose MV strategy at 4 and 9 months reduces all-cause mortality between 4 and 60 months of age by 30%, and 2) receiving MV at 4 months in the presence versus absence of MatAb reduces child mortality by 35%. We found no beneficial effect of a 2-dose compared to a 1-dose strategy on all-cause mortality. Mortality was 50% (20–68%) lower for those children vaccinated in the presence of MatAb compared with those vaccinated in absence of MatAb.
Implications of all the available evidence
The findings suggest that early MV in the presence of MatAb would both protect more infants from measles infection and reduce all-cause mortality. Further research should explore whether it would be beneficial to provide MV to women of fertile age to enhance antibody transfer to their children.
Alt-text: Unlabelled box
Introduction
When measles vaccine (MV) was introduced in low-income countries a major decline in mortality occurred.1, 2, 3 The reduction was larger than explained by protection against measles; this led to the hypothesis that MV had beneficial non-specific effects (NSEs).4 In the 1990s, we therefore pursued the idea that an additional early dose of MV could further reduce mortality.5,6 The results were not clear-cut. The 3rd dose of diphtheria-tetanus-pertussis (DTP3) vaccine was scheduled at 14 weeks of age, but many received DTP after MV. Early MV was not beneficial if DTP was given after MV.7
Subsequently, in a randomised controlled trial (RCT), we examined early MV when DTP was not administered after MV.8 Children who received DTP3 four weeks prior to enrolment were randomised to MV at 4.5 and 9 months (2-dose), or MV at 9 months (1-dose). The per-protocol (PP) mortality hazard ratio (HR)(2-dose/1-dose) was 0.70 (95%CI: 0.52–0.94) between 4.5–36 months of age.
Intriguingly, children who received early MV in presence of maternal measles antibody (MatAb) had much lower mortality than children vaccinated in absence of MatAb.9
MV is recommended when MatAb has waned because MatAb lowers the serological response.10,11 Hence, high-income countries vaccinate at 12–18 months of age. Giving MV at 9 months in low-income countries is a compromise to reduce infant measles. Once measles is “under control”, WHO recommends raising vaccination-age to 12 months. The observation that MV in presence of MatAb is associated with lower mortality challenges current policies12 and needs to be repeated.
In 2011, we therefore initiated another RCT of early MV and all-cause mortality. We collected blood samples at enrolment to study the effect of receiving MV in presence or absence of MatAb.
Our hypotheses were 1) a 2-dose strategy at 4 and 9 months reduces mortality between 4 and 60 months of age by 30%, and 2) receiving MV at 4 months in the presence versus absence of MatAb reduces child mortality by 35%.
Methods
Study design
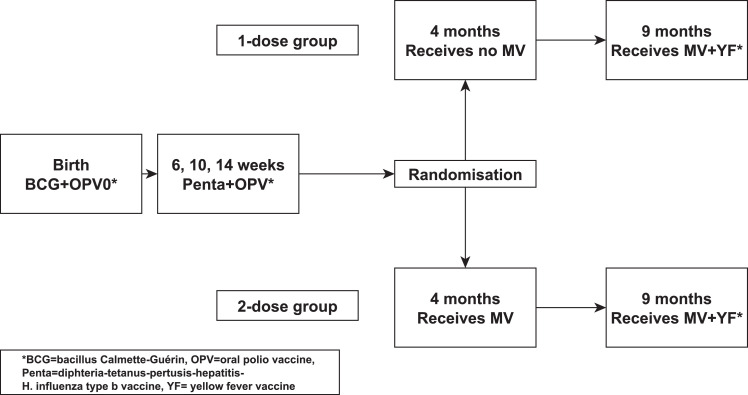
Single-centre open-label community-based RCT of early MV vs. no early MV at 4 months of age; all children received the recommended MV at 9 months (Figure 1). The two main objectives were: First, assess whether an early 2-dose schedule reduces all-cause mortality. Second, examine whether receiving MV in presence of MatAb lowers all-cause mortality. The National Ethical Committee in Guinea-Bissau approved the protocol, and the Danish Central Ethical Committee gave consultative approval. We adhered to the CONSORT guidelines for reporting of randomized controlled trials.
Figure 1.
Trial design for the 2-dose versus 1-dose measles vaccine (MV) trial.
Participants
The RCT was initiated in August 2011 at Bandim Health Project (BHP), Guinea-Bissau (www.bandim.org), a Health and Demographic Surveillance System site, which covers six districts with 100,000 inhabitants.
Residents have a unique identification number in the databases; socio-economic and demographic information can be retrieved from these databases. Houses are visited monthly to register new pregnancies and deliveries. amongst women of fertile age, 7% are HIV-1 or HIV-2 positive.13 Information on birth-weight is not available for all, but at the national hospital approx. 16–17% of BHP children have a birth-weight <2500 g (unpublished data).
Children aged 0–2 years are visited at home every 3–4 months to collect information on vaccinations, breastfeeding, infections, hospitalisations, and survival. Three health centres provide routine vaccinations in the BHP area. In 2011, the vaccination program was bacillus Calmette-Guérin (BCG)+oral polio vaccine (OPV) at birth, three doses of DTP-containing pentavalent vaccine (Penta) plus OPV at 6, 10 and 14 weeks, and MV and yellow fever vaccine at 9 months of age. Children from the BHP area aged 4–7.5 months were eligible four weeks after Penta3.
We previously observed no effect of early MV after neonatal vitamin A supplementation (NVAS).8 NVAS is not routine in Guinea-Bissau, but we asked for and excluded children who had received NVAS (n = 0). Children with severe malformations, needing hospitalisation or severely malnourished (mid-upper-arm-circumference<115 mm) were not enroled but referred for treatment. These children were enroled if they recovered before 7.5 months of age and fulfilled enrolment criteria.
The enrolment team consisted of a study physician, nurses, and field workers. The team worked at the three health centres on different weekdays. Mothers of eligible children were visited in the morning, received a study explanation, answered a short questionnaire, and were invited to bring the child in the afternoon. Here, the mothers received an oral and a written explanation from the physician: the best MV strategy was not known and BHP therefore conducted the trial. Participation was voluntary. Mothers/guardians were asked to provide oral and written consent. The physicial performed a medical examination.
Randomisation and masking
Block randomisation 2:1 to receive early or no early MV, with 24 envelopes per bag, was performed separately for males and females. Envelopes were prepared by the supervisor. Same-sex twins drew one envelope and received the same treatment to avoid confusion about which twin received what.
The enrolment team and mothers were not blinded regarding treatment. Mothers in the 2-dose group were informed that their child would receive early MV now and be invited back for MV at age 9 months. Mothers in the 1-dose group were told that their child did not receive MV now but would be invited to receive MV at age 9 months.
A control vaccine could not be used since we intended to measure the NSEs of MV.6,8,14 Had we used placebo, some mothers might erroneously have believed that their child had received MV. If they moved, they might not have sought MV.
We did not record the 4-month MV on the vaccination card. Thus, field assistants and staff at health centres and paediatric wards were blinded.
Procedures
We used standard Edmonston-Zagreb MV from Serum Institute of India. According to quality tests, the mean virus concentration for trial batches ranged from log10 3.67 (95%CI: 3.38 to 3.95) to 3.93 (3.79 to 4.08) CCID50/0.5 ml. As per standard, 0.5 ml vaccine injection was administered subcutaneously in the scapular region.
Children were followed at scheduled health centre visits at 9, 36 and 60 months of age. If children were travelling at 9 months, we kept visiting them until they were 18 months old. The children were also visited at home every 6 months. We obtained information about vital status and migration. Children moving within the BHP area were followed to their new address. Children moving out of the area were censored at moving.
Following a death, a trained field assistant visited the household to conduct a verbal autopsy.15 Cause of death was assigned by a physician reviewing the information.
Twenty-two national campaigns were conducted during the study. Trial participants were exempted from MV-campaigns in 2012 and 2015, but not in the last MV-campaign in May 2019.
The twenty-two campaigns included OPV (n = 11, age group 0–59 months), vitamin A supplementation (n = 16, 6–59 months) or mebendazole (n = 16, 12–59 months) (Supplementary Table 1).
Blood samples
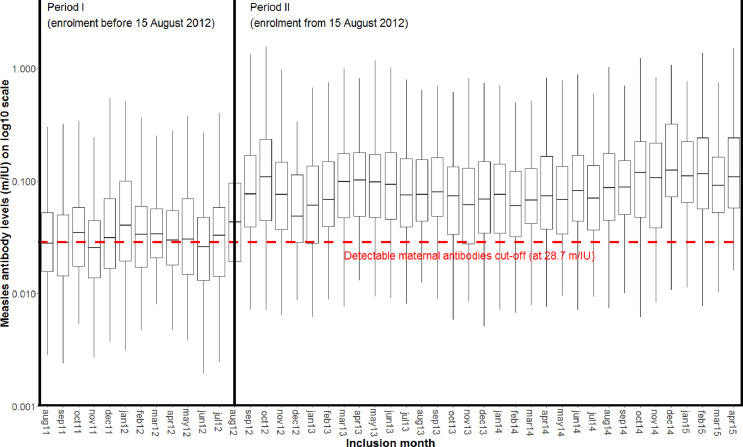
Blood samples were collected at enrolment; serum was separated within a few hours and frozen at minus 20 °C. Samples were subsequently transported to the National Institute of Public Health and the Environment, The Netherlands, to determine measles antibody levels. We used bead-based multiplex immunoassay (MIA) which correlates well with both plaque reduction neutralization test and haemagglutination inhibition (HAI) assays.16 We also assessed varicella, mumps and rubella antibodies. We previously showed that a MIA-result of 28.7 mIU/mL corresponds to detectable antibody in the HAI test.16 We used this value as cut-off for presence of MatAb.
In the analysis, it became apparent that the measles antibody level had changed around mid-August 2012 (Figure 2). Similar changes occurred for varicella and mumps antibody, but not for rubella (probably due to a small rubella-epidemic)(Supplementary Figure 1). Despite extensive searches we could not identify an explanation for this sudden jump in levels: it did not coincide with changes in staff, the handling of tubes and centrifugation was constant throughout the trial, there were no freezer breakdowns, and no thawing of samples during transport. The sample preparation and analysis in the Netherlands was done identical for all samples. Since samples were analysed in mixed batches covering different time periods of collection during a short period in the Netherlands, it is most likely that the explanatory change/event occurred in Guinea-Bissau.
Figure 2.
Levels of maternal measles antibodies by Study Period (before and after August 15, 2012).
Outcomes
The primary outcome was all-cause mortality measured in the per-protocol population. Prespecified secondary outcomes have been or will be reported elsewhere.
Statistical analysis
We anticipated 5.6% mortality risk between 4 and 60 months of age.8 With 6600 children randomised 2:1 to intervention and control groups, we could show 30% lower mortality in the 2-dose MV group as seen in the previous trial,8 and 35% lower mortality for early MV in presence vs. no presence of MatAb with 80% power and a 5% significance level.
Children were followed until they migrated, died, turned 60 months or May 3, 2019 (national MV campaign), whichever came first. A per-protocol estimate was obtained by censoring children, who deviated from the planned vaccination schedule (received MV elsewhere, did not receive 9-month MV before 18 months of age).8
We present deaths and observation time together with hazard rate ratios (HR) and Wald 95% CIs estimated from a Cox proportional hazards model, with age as underlying time. Age was inherently adjusted for; we also adjusted by stratification for residential district and sex.8 Since same-sex twins were not individually randomised, we adjusted CI-estimates by clustering of same-sex twin pairs. The intention-to-treat (ITT) analysis included extra follow-up time from 338 children (1 death), who did not receive 9-month MV from us (Supplementary material).
The proportional hazards assumption was assessed graphically and tested using Schoenfeld residuals; the assumption was rejected in the analysis between 4 and 60 months of age (p = 0.045). Adding the time-varying covariate “number-of-OPV-campaigns-received” to the model solved the pH violation and only changed results very little (+/- 0.01 for estimates and CIs). For the remaining analyses the assumption was not rejected, and no additional changes were made to the models.
Since antibody levels before and after August 15, 2012 (Period-I and Period-II) were stable for measles, varicella and mumps, we assume that these values represent consistent measurements in two different periods. In the analyses of the whole period, we stratified by Period, allowing for different baseline hazard functions for the two Periods.
To assess impact of mumps, rubella and varicella maternal antibodies, we calculated HRs amongst children with detectable measles MatAb, comparing the three upper quartiles of each antibody against the lower quartile, as there was no known cut-off for detectable antibody for these antigens. Otherwise, analyses were performed the same way as described above for MatAb.
The Kaplan-Meier method was used to calculate accumulated mortality curves for the 2-dose and 1-dose MV groups. The Number Needed to Treat (NNT) was calculated as the inverse of the absolute risk difference in the Kaplan-Meier survival estimates.
Effect modification. As NSEs may be sex-differential, we prespecified that all analyses be done overall and by sex. In the previous trial, we observed interaction between early MV and campaigns with OPV (C-OPVs).17 Therefore, we investigated the interaction with C-OPV in the present trial. Information about C-OPV was obtained by inspection of the vaccination card and by asking the mother at enrolment (information obtained from 99.8%). The information obtained at enrolment was used to assess the impact of campaigns before enrolment on subsequent mortality. As we could not get information on campaign participation after enrolment for all children and allocation could affect campaign participation, and as we know that campaign participation rates are usually above 90%,18 in the analysis of campaigns-after-enrolment, we assumed that all eligible children received C-OPV during OPV campaigns. Thus, a given child would be contributing risk time as “Not received or not yet received C-OPV-after-enrolment” from enrolment and until a national campaign occurred, after which it would contribute time as “Received C-OPV-after-enrolment”. Potential interactions with other campaigns were examined in a similar manner.
We also investigated effect modification by season (of enrolment and during follow-up; rainy=June-November; dry=December-May).8 Secondary analyses are further explained in the Supplementary material.
Statistical analyses were conducted with Stata 16 and 17.
Trial registration: NCT01486355.
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. SN, PA and CSB had access to the dataset and final responsibility for decision to submit for publication.
Results
Study population
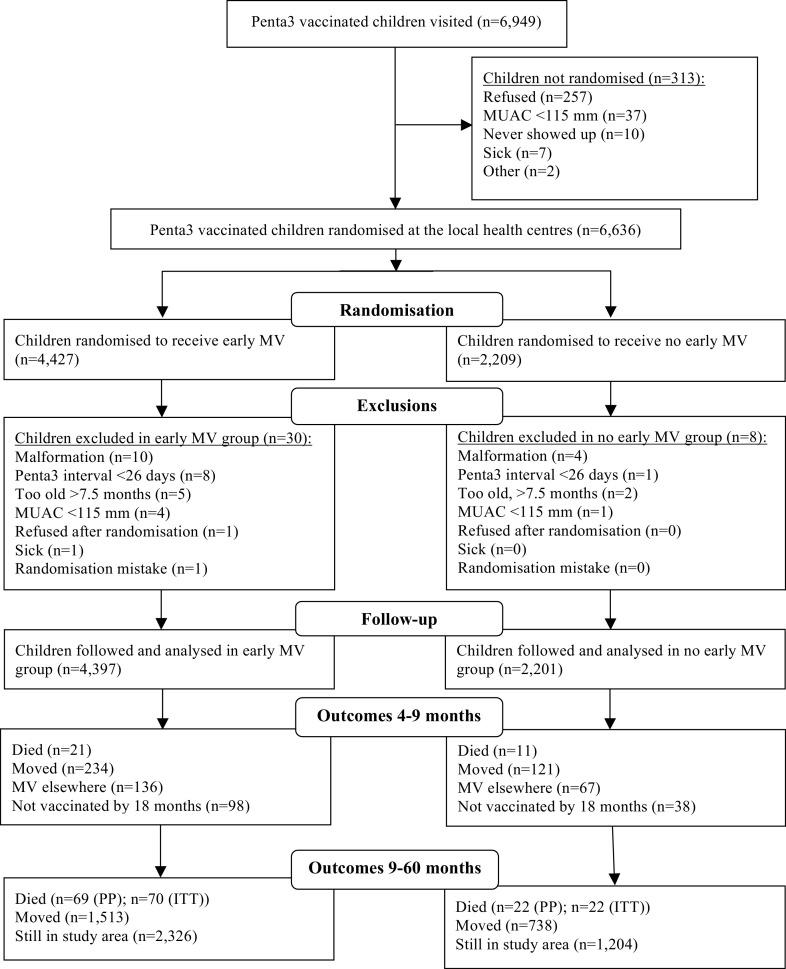
From August 2011 to April 2015, we enroled 6636 children, 6598 of whom were included in the analysis; 4397 in the 2-dose group and 2201 in the 1-dose group (Figure 3). There were no major differences in background factors (Table 1).
Figure 3.
Trial diagram for the 2-dose versus 1-dose measles vaccine (MV) trial in Guinea-Bissau, 2011–2019.
Table 1.
Background factors for the 2-dose measles vaccine (MV) group and 1-dose MV group. Values are percentages (numbers) unless stated otherwise.
| 2-dose group (n = 4397) | 1-dose group (n = 2201) | |
|---|---|---|
| Demographic factors | ||
| Median (interquartile range) age at enrolment (months) | 4.9 (4.7–5.4) | 4.9 (4.7–5.4) |
| Median (interquartile range) age of mother (years) | 25 (22–30) | 26 (22–30) |
| Bandim district | 43 (1909) | 43 (945) |
| Male sex | 52 (2302) | 52 (1151) |
| Twins | 3.2 (141) | 3.1 (69) |
| Pepel ethnicity | 27 (1184) | 27 (598) |
| Mother had died (number) | 6 | 5 |
| Risk factors at enrolment | ||
| Not breastfed at 4 months | 1.1 (47) | 1.0 (23) |
| Pigs in household | 12 (536) | 13 (277) |
| Number of people/bed | 2.9 | 2.9 |
| Number of people/sleeping room | 4.3 | 4.3 |
| Toilet inside house | 21 (932) | 21 (462) |
| Functioning electricity | 34 (1476) | 34 (743) |
| Sleep under mosquito net | 99 (4357) | 99 (2174) |
| Maternally reported morbidity and anthropometry at enrolment | ||
| Hospital admission before enrolment | 5.4 (236) | 5.0 (111) |
| fever | 22 (964) | 21 (472) |
| diarrhoea | 9.8 (429) | 9.8 (216) |
| BCG scar | 92 (4064) | 93 (2040) |
| Have antimalarials at home | 8.7 (381) | 8.1 (177) |
| Mean (SD) weight (g) | 7192 (973) | 7187 (963) |
| Mean (SD) arm circumference (mm) | 141 (11) | 141 (11) |
| Mean (SD) height (cm) | 63 (2.7) | 63 (2.7) |
| Mean (SD) mother's arm circumference (mm) | 281 (38) | 283 (38) |
| Medication received within 3 days of enrolment | ||
| Any medication | 21 (933) | 22 (474) |
| Paracetamol | 16 (701) | 16 (361) |
| Anti-malarial | 4.2 (185) | 3.9 (85) |
| Antibiotics | 9.4 (412) | 9.3 (205) |
| Campaign OPV (C-OPV) before enrolment: participation prevalence amongst all eligible children (participants / eligible children) | ||
| First OPV campaign 2011 | 55 (53/97) | 40 (23/57) |
| Second OPV campaign 2011 | 70 (157/225) | 58 (70/120) |
| Third OPV campaign 2011 | 74 (437/592) | 75 (217/290) |
| OPV campaign 2012 | 40 (238/598) | 44 (130/296) |
| First OPV campaign 2013 | 50 (253/507) | 53 (137/260) |
| Second OPV campaign 2013 | 52 (242/462) | 53 (132/248) |
| First OPV campaign 2014 | 48 (196/411) | 51 (101/198) |
| Second OPV campaign 2014 | 53 (204/383) | 64 (118/184) |
| C-OPV-before-enrolment participation amongst all eligible trial children | 61 (1595/2618) | 63 (836/1335) |
| C-OPV-before-enrolment participation amongst all trial children | 36 (1595/4397) | 38 (836/2201) |
Valid blood samples for assessment of MatAb were obtained from 95% (6239) (Supplementary Figure 2); 53% (951/1798) had detectable MatAb in Period-I and 84% (3735/4441) in Period-II (Supplementary Table 2).
During follow-up, 123 children died (ITT-analysis: 124) and the mortality risk before 5 years of age was 1.95%. Between 4–9 months, 32 children died (mortality risk=0.5%). The PP-analysis between 9 and 60 months had 5872 children (ITT-analysis: 6211) (Table 2, Supplementary Table 3).
Table 2.
Per protocol (PP) mortality rates and hazard ratios (HR) (with 95% CI) between the 2-dose measles vaccine (MV) group and the 1-dose MV group amongst all children (n = 6598) in the analysis between 4 and 60 months of age.
| Mortality rates per 100 person years (deaths/person years) |
HR (2-dose/1-dose) (95% CI) #1 | ||
|---|---|---|---|
| 2-dose group (n = 4397) | 1-dose group (n = 2201) | ||
| 4–9 months | |||
| Males | 0.90 (8/887) | 1.37 (6/436) | 0.66 (0.23–1.89) #2 |
| Females | 1.65 (13/786) | 1.28 (5/391) | 1.29 (0.46–3.62) #2 |
| All | 1.25 (21/1674) | 1.33 (11/828) | 0.94 (0.45–1.96) |
| 9–60 months (n = 5872) | |||
| Males | 0.66 (42/6369) | 0.39 (13/3291) | 1.66 (0.89–3.09) #3 |
| Females | 0.45 (27/6013) | 0.30 (9/2997) | 1.49 (0.70–3.16) #3 |
| All | 0.56 (69/12,382) | 0.35 (22/6288) | 1.60 (0.99–2.59) |
| 4–60 months | |||
| Males | 0.69 (50/7250) | 0.51 (19/3724) | 1.33 (0.78–2.26) #4 #5 |
| Females | 0.59 (40/6793) | 0.41 (14/3386) | 1.42 (0.77–2.60) #4 #5 |
| All | 0.64 (90/14,043) | 0.46 (33/7110) | 1.38 (0.92–2.06) #4 #5 |
#1 HR (95% CI) estimated in a Cox-proportional hazards model with age as underlying time scale. #2 Test of interaction, p = 0.37; #3 Test of interaction, p = 0.83; #4 Test of interaction, p = 0.88; #5 Model with added covariate “Number of campaign-OPVs received” to avoid rejection of proportional hazards assumption in the Cox model.
Seven study children (2-dose: 4; 1-dose: 3) were diagnosed with measles infection at health centres (n = 6) or hospital (n = 1). There was no epidemic links, occurring in five different years and with no geographical clustering. During the trial, four samples from suspected measles infection, including two trial children, were tested for IgM at the national laboratory. All samples were IgM negative. Hence, we have assumed that the suspected cases were wrongly diagnosed. No suspected case died.
Main results
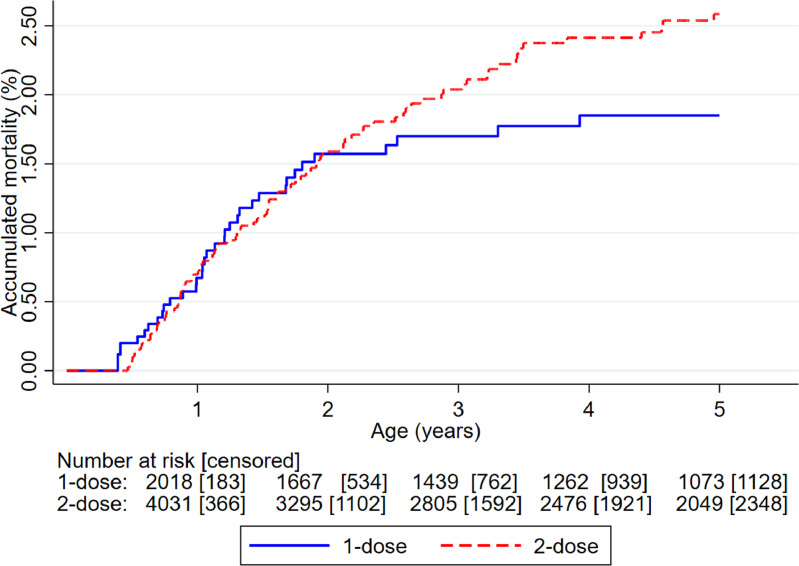
The HR(2-dose/1-dose) was 1.38 (0.92–2.06); 1.33 (0.78–2.26) for males and 1.42 (0.77–2.60) for females (Table 2, Figure 4). Between 4–9 months, with 1 dose MV-after-Penta3 vs. Penta3 as most recent vaccination, the HR(1-dose/0-dose) was 0.94 (0.45–1.96). Between 9–60 months, the HR(2-dose/1-dose) was 1.60 (0.99–2.59) (Table 2).
Figure 4.
Kaplan-Meier curves of accumulated mortality in children randomised to 2-dose versus 1-dose measles vaccine (MV).
There was non-significantly higher mortality in the 2-dose group for diarrhoea, malaria and other infectious diseases, but slightly lower mortality for pneumonia/respiratory infections (Supplementary Table 4). Censoring seven accident deaths (all in the 2-dose group), the HR(2-dose/1-dose) was 1.27 (0.85–1.90). There were no measles deaths.
Interactions with season and other interventions
The effect of early MV did not vary by season (Supplementary Table 5). For other interventions, we determined the HR(2-dose/1-dose) before and after campaigns (Supplementary Table 6). Only OPV-campaigns had a significant effect on results (p for interaction=0.027).
Interaction with campaign-OPV
C-OPV was the only intervention given both before and after enrolment. Participation was similar in both randomisation groups (Table 1). Children were enroled over a long period, and C-OPVs were not regular, so children could receive C-OPV before-enrolment, after-enrolment, before-and-after-enrolment or no C-OPV. The HR(2-dose/1-dose) for children who had not received C-OPV-before-enrolment was 1.05 (0.65–1.68) compared to 2.43 (1.13–5.22) amongst recipients of C-OPV-before-enrolment (p for interaction=0.065) (Table 3). Among children who received C-OPV before-and-after-enrolment the HR(2-dose/1-dose) was 4.73 (1.44–15.6) as compared with 0.81 (0.29–2.22) for children who received no C-OPV (p for interaction=0.027). Further analyses by C-OPV status revealed that the increased mortality in the 2-dose group associated with C-OPVs was linked to an increase in female mortality following C-OPV-before-enrolment, and lack of beneficial effects of subsequent boosting with C-OPVs, as strongly present in the 1-dose group (Supplementary Table 6).
Table 3.
The mortality rates and hazard ratio (HR) of children in the 2-dose measles vaccine (MV) group compared with 1-dose MV group in relation to the administration of campaign OPV (C-OPV) before enrolment and C-OPV-after-enrolment between 4 and 60 months.
| Received no C-OPV-before-enrolment |
Received C-OPV-before-enrolment |
|||||
|---|---|---|---|---|---|---|
| Mortality rates per 100 person years (deaths / person years) |
HR (2-dose/1-dose) (95% CI) #1 | Mortality rates per 100 person years (deaths / person years) |
HR(2-dose/1-dose) (95% CI) #1 | |||
| 2-dose group | 1-dose group | 2-dose group | 1-dose group | |||
| Not received or not yet received C-OPV-after-enrolment | ||||||
| Males | 1.38 (7/509) | 1.62 (4/246) | 0.85 (0.25–2.91) #2 | 0.48 (2/418) | 1.83 (4/219) | 0.26 (0.05–1.44) #3 |
| Females | 0.60 (3/496) | 0.85 (2/236) | 0.72 (0.12–4.33) #2 | 2.24 (8/357) | 0.54 (1/186) | 4.11 (0.52–32.6) #3 |
| All | 0.99 (10/1005) | 1.24 (6/482) | 0.81 (0.29–2.22) | 1.29 (10/775) | 1.23 (5/405) | 1.05 (0.36–3.05) |
| Received C-OPV-after-enrolment | ||||||
| Males | 0.63 (24/3812) | 0.47 (9/1935) | 1.34 (0.62–2.88) #4 | 0.68 (17/2512) | 0.15 (2/1324) | 4.46 (1.03–19.3) #5 |
| Females | 0.51 (19/3760) | 0.56 (10/1793) | 0.92 (0.43–1.98) #4 | 0.46 (10/2180) | 0.09 (1/1171) | 5.17 (0.66–40.3) #5 |
| All | 0.57 (43/7571) | 0.51 (19/3728) | 1.12 (0.65–1.93) | 0.58 (27/4692) | 0.12 (3/2495) | 4.73 (1.44–15.6) |
| Combined | ||||||
| Males | 0.72 (31/4320) | 0.60 (13/2181) | 1.19 (0.62–2.27) #6 | 0.65 (19/2930) | 0.39 (6/1543) | 1.66 (0.66–4.17) #7 |
| Females | 0.52 (22/4256) | 0.59 (12/2029) | 0.89 (0.44–1.79) #6 | 0.71 (18/2537) | 0.15 (2/1357) | 4.64 (1.08–20.0) #7 |
| All | 0.62 (53/8576) | 0.59 (25/4210) | 1.05 (0.65–1.68) | 0.68 (37/5467) | 0.28 (8/2901) | 2.43 (1.13–5.22) |
#1 HR (95% CI) estimated in a Cox-proportional hazards model with age as underlying time scale. #2 Test of interaction, p = 0.88; #3 Test of interaction, p = 0.045; #4 Test of interaction, p = 0.50; #5 Test of interaction, p = 0.91; #6 Test of interaction, p = 0.55; #6 Test of interaction, p = 0.24.
Explorative analysis: the timing of Penta3 and early MV
Since a previous study showed higher mortality for children receiving MV within 4 weeks after DTP323, we tested whether the timing of Penta3 and MV had an impact. The effect of early MV tended to improve with increasing interval between Penta3 and MV; for each additional week between Penta3 and early MV, the HR(2-dose/1-dose) decreased by 0.75 (0.50–1.12)(Supplementary Table 7).
Impact of MatAb
A total of 53% (951/1798) had detectable MatAb in Period-I and 84% (3735/4441) in Period-II (Supplementary Table 2). As expected, detectable antibody levels declined rapidly between 4 and 7 months of age. As there was no confirmed measles case during the trial, all antibody detected are likely to represent maternal antibody. Very few background factors affected the likelihood of having detectable MatAb. Children with no detectable MatAb had marginally younger mothers (Period-I, Period-II). They were more likely to have been hospitalised before enrolment (Period-II), to be enroled in the rainy season (Period-II), and to have a higher weight at enrolment (Period-I)(Supplementary Table 8).
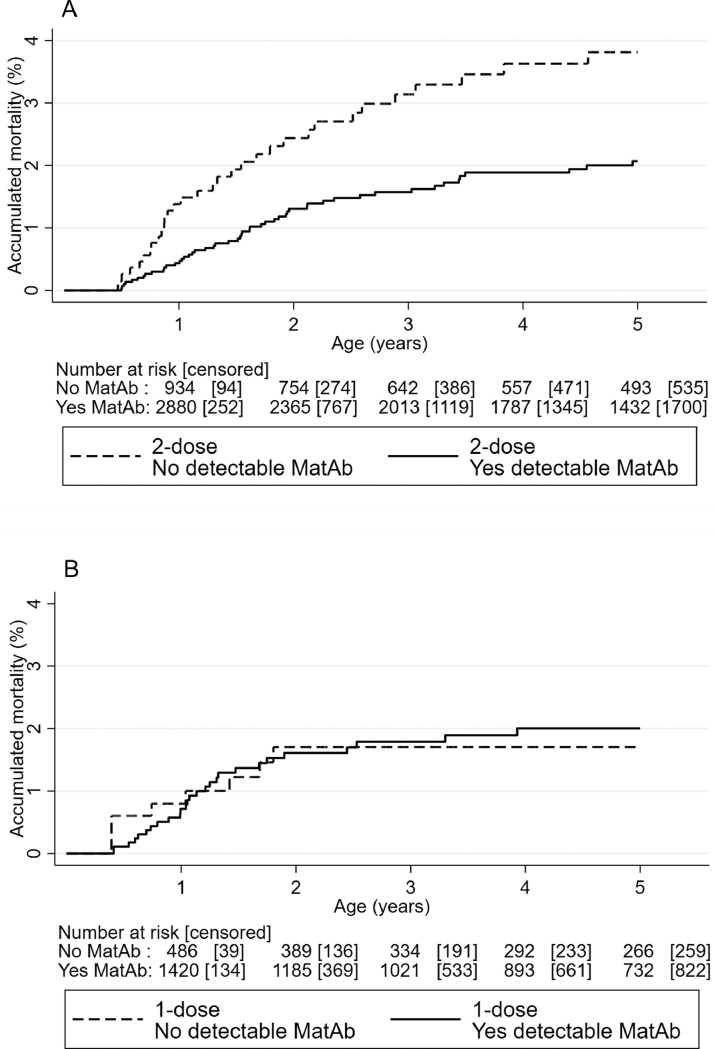
Early MV group (2-dose). In the 2-dose group, all-cause mortality was lower amongst children who received early MV in presence of MatAb (n = 3132; 51 died) compared with no MatAb (n = 1028, 31 died), the HR(MatAb/noMatAb) being 0.50 (0.32–0.80)(Table 4, Figure 5). The difference was most marked between enrolment and the 9-month MV (HR(MatAb/noMatAb)=0.32 (0.13–0.76)) but still apparent after 9-month MV and until 5 years of age (HR(MatAb/noMatAb)=0.60 (0.34–1.04))(Table 4). Adjusting for determinants of MatAb did not change results (data not shown).
Table 4.
Mortality rates and hazard ratios (HR) comparing children with detectable measles maternal (MatAb) and no detectable MatAb by time of enrolment. Follow-up from enrolment to the 9-month measles vaccine (MV) and after 9-month MV to end of study.
| Mortality rates per 100 person-years (deaths/person-years) (n=number of children) |
HR (detectable/no detectable MatAb) (95% CI) #1 | ||
|---|---|---|---|
| Detectable MatAb #2 | No detectable MatAb #2 | ||
| Early MV group; received MV at 4 months | |||
| Before 9-month MV (n = 4160) | 0.75 (9/1197) (n = 3132) | 2.65 (10/377) (n = 1028) | 0.32 (0.13 to 0.76) |
| After 9-month MV (n = 3703) | 0.47 (42/8895) (n = 2802) | 0.74 (21/2826) (n = 901) | 0.60 (0.34 to 1.04) |
| Combined (n = 4160) | 0.51 (51/10,086) (n = 3132) | 0.97 (31/3199) (n = 1028) | 0.50 (0.32 to 0.80)#3 |
| No early MV group; received no MV at 4 months | |||
| Before 9-month MV (n = 2079) | 1.38 (8/581) (n = 1554) | 1.53 (3/196) (n = 525) | 0.94 (0.23 to 3.80) |
| After 9-month MV (n = 1855) | 0.40 (18/4457) (n = 1389) | 0.20 (3/1466) (n = 466) | 1.86 (0.55 to 6.21) |
| Combined (n = 2079) | 0.52 (26/5033) (n = 1554) | 0.36 (6/1662) (n = 525) | 1.39 (0.57 to 3.38)#3 |
#1 The Cox models were stratified by Period; #2 Detectable MatAb cut-off defined at MIA >28 mIU; #3 P-value for test of interaction=0.045.
Figure 5.
Kaplan-Meier curves of accumulated mortality in children randomised to 2-dose measles vaccine (MV) (A) and in children randomised to 1-dose MV (B) by maternal measles antibody (MatAb) levels.
The association between MatAb and mortality was similar in Period-I and Period-II and whether the children had received C-OPV before enrolment or not (Supplementary Table 9). Also, it was not affected by age at enrolment (Supplementary Table 10). Both infectious and non-infectious causes of death tended to be lower amongst children with detectable MatAb, but accidents were not (Supplementary Table 11). Higher levels of other antibodies were not associated with lower mortality after early MV (Supplementary Table 12).
No early MV group (1-dose). Amongst children receiving no early MV, mortality was not lower for children with detectable MatAb than for those without. In fact, the trend was in the opposite direction (HR (MatAb/noMatAb)=1.39 (0.57–3.38))(p for interaction between randomisation group and MatAb=0.045) (Table 4, Figure 5).
Impact
The NNT with early MV in presence of MatAb to save one child from dying at 4–60 months of age was 57 (95%CI=28–283).
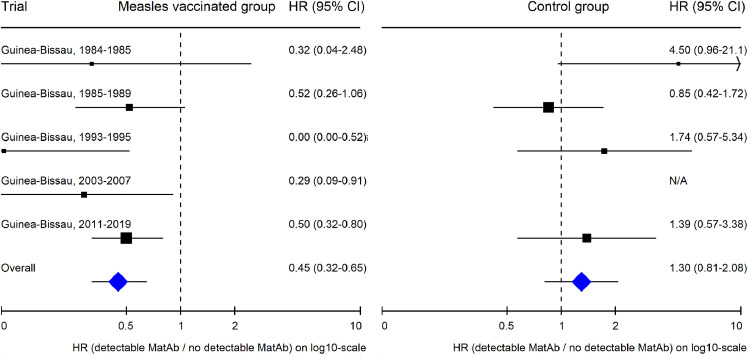
Meta-analysis
Five studies, all from Guinea-Bissau, examined the potential effect of having MatAb when being vaccinated with MV (Figure 6, Supplementary Table 13).9,19 A combined analysis found 55% (35–68%) lower mortality up to age 5 years for those who received MV in the presence versus the absence of MatAb. In the four studies where MatAb was also measured in control groups that did not receive MV, the tendency was opposite with 30% (−19–108%) higher mortality up to age 5 years for those who had MatAb (p for interaction<0.0001).
Figure 6.
Meta-analysis: Comparing overall survival of children with vs. without maternal measles antibody (MatAb) in measles-vaccinated children and in control children unvaccinated against measles, respectively.
Discussion
Contrary to our first hypothesis,8 early MV had no beneficial effect on all-cause mortality. Numerous C-OPVs may have played a role: Early MV tended to be beneficial if children had received no C-OPVs, but negative if children had received C-OPV-before-and-after-enrolment. Supporting this interpretation, we previously observed a similar negative interaction between early MV and C-OPV.17
Supporting our second hypothesis and corroborating four previous studies,9,19 having MatAb when receiving MV was associated with reductions in mortality. This effect was specific to the combination of early MV in presence of MatAb; no effect of MatAb was seen in children, who did not receive early MV (control group), and no effect of other maternal antibodies was seen in children, who received early MV (control exposure). The NNT to save one child by providing early MV in the presence of MatAb was a mere 57.
The randomisation generated no major differences in background factors and in exclusions and drop-outs. The participants were not blinded, but those involved in the follow-up were blinded regarding the children's vaccination status. In theory, parents’ health care seeking behaviour could be affected by their lack of blinding but based on our experience caregivers do bring their children to consultations if ill, irrespective of vaccination status.
Unfortunately, we had two periods with rather different antibody levels. There was no simple explanation. Within each period the antibody levels were consistent for both measles and other antibodies. The change in antibody level presumably meant that in Period-I, some children with detectable antibody levels may have been misclassified as having no antibody. Nonetheless, the beneficial effect of MatAb at measles vaccination was essentially the same in both Periods.
Mortality was 65% lower than expected. A major contributing factor may have been the OPV campaigns.18,20 Further factors could be the introduction of rotavirus vaccine in 2012 and the introduction of pneumococcal vaccine in 2015. The latter did not affect trial participants but may have reduced the community level of pneumococcal infections. With the observed mortality and the number of participants, we only had 35% power to detect a 30% reduction in all-cause mortality after early MV.
There were no laboratory-confirmed measles cases during the trial and no measles deaths. Hence, effects of MV would be non-specific to measles infection.
NSEs of vaccines may be due to altered programming of immunity, e.g. through epigenetic changes induced by the vaccine.21, 22 From this perspective, we need to consider the potential interactions with other immune-stimulatory interventions. We had previously observed that DTP-after-MV7 and NVAS-before-enrolment8 influence early MV-effects; this knowledge was built into the design. A key variable was “C-OPV-before-enrolment”.17 This information was based on the child's vaccination card at enrolment. Some degree of misclassification may have occurred. Non-registration of receiving C-OPV-before-enrolment would reduce the estimated differential effect of C-OPVs.
It would be logistically impossible to conduct an RCT where children with known MatAb status were randomised to early MV, as there was no rapid-test for measles antibody level. However, by design, we tested the effect of MatAb versus no MatAb in groups randomised to early MV or no early MV; hence, the no-early-MV-group was a control group. Having MatAb was only associated with lower mortality in the early-MV-group, not in the control group. Thus, the results cannot be explained by differences between children with and without MatAb. Furthermore, presence of higher levels of other maternal antibodies than measles was not related to mortality. The use of a control group and control exposures supported that the survival effect was linked specifically to MV in the presence of MatAb. The effect of MatAb was seen independent of C-OPV status.
This is the second RCTs to find no benefit of early MV,23 and it could be speculated that early MV has no beneficial NSEs. However, overall effects and female mortality in the present trial differed significantly from the previous RCT in Bissau8 but were similar to another RCT conducted simultaneously in the rural area.23 Results are therefore unlikely to be random variation around a “true” null-effect, but rather reflect that interacting factors may have produced divergent outcomes.8,23
The conflicting results are compatible with a negative interaction between C-OPV and early MV as the number of C-OPVs increased between the first trial8 and the latter trials.23 Also, in a post-hoc analysis of the first trial, we showed that effects of early MV differed by C-OPV status as in the present trial17; data for such an analysis was unfortunately not available for the rural trial. Morbidity and pneumococcal data from the trial supported similar interactions between MV and C-OPV (Supplementary material). C-OPVs are associated with substantial reductions in mortality, with additional reductions per additional C-OPV.18,20 Each additional C-OPV reduced the mortality level in the 1-dose group but had limited beneficial effect on the mortality rate in the 2-dose group. We know from other studies that the sequence or combination of early life vaccines may impact subsequent mortality.7 The underlying biological mechanisms have not been studied.
Other factors than C-OPV may have contributed to finding no beneficial effect of early MV. Simultaneous DTP+MV vaccinations have negative effects for survival7 and similar to a previous study23 the present trial suggests that a short interval between Penta3 and MV may also increase mortality; for optimal early MV effect, the interval may need to be 6 rather than 4 weeks.
The present study is consistent with the four previous reports of strongly beneficial effects of MV in presence of MatAb.9,19 No study has reported the opposite trend. Noteworthy, beneficial effects of maternal priming and subsequent child vaccination with the same live vaccine have also been seen for BCG.24, 25
The five studies cover a period of 35 years. In the first studies, nearly all MatAb would have been generated by natural infection. MV was implemented in Guinea-Bissau in 1986. In 2006 there was a national MV campaign for all under age 15 years. Hence, those born after 1990 and a major part of those born in the 1980s, like mothers in the present trial, would have received MV.
The mechanisms explaining beneficial effects of MV in presence of MatAb are unknown. Several mechanisms, not mutually exclusive, could be important, e.g. MV in the presence high-affinity maternal antibody would lead to responses to subdominant epitopes, more diverse T- and B-cell repertoires and increased heterologous protection against other pathogens.9 The benefits of MV in presence of MatAb was most marked before 9 months of age, i.e. before children without detectable MatAb received the second MV. Presumably, for children with no detectable MatAb at enrolment, antibody response to the first dose may have affected the response to 9-months dose like MatAb did, thus diminishing the difference between children with and without MatAb.26 Hence, repeated infant immunizations with the same live vaccine may also generate beneficial effects.
Five studies have now found marked reductions in mortality when MV is given in the presence of MatAb.9,19 These observations question the rationale behind the current MV strategy. MV policies avoid MV in presence of MatAb due to fear of blunting and lower antibody responses.11,27 However, as shown earlier, even though an early 2-dose strategy may result in reduced antibody levels by 24 months of age, it does not impair seroconversion and importantly, early MV protected children against measles hospitalisations and deaths in infancy.27 Furthermore, the only study linking measles antibody level to subsequent mortality found that lower measles antibody levels were associated with lower, not higher, mortality over the next 5 years.28
The available data thus supports that if we could give early MV in presence of MatAb, it would not hamper measles control and it would lead to lower overall mortality.
Worryingly, we observed that in a context with many C-OPV, early MV might be harmful if given to children with no MatAb – a situation that would be impossible to avoid in practical terms. MV offered at 9 months may not interact negatively with C-OPV, but it seems necessary to clarify this issue. To proceed safely, while increasing our knowledge about the NSEs of both C-OPV and MV, we need a large multi-centre trial to explore interactions between C-OPV and MV in the current schedule.
In conclusion, contrary to the first RCT,8 early MV did not benefit survival. We identified interactions with C-OPVs that may have modified the early MV effect. As part of the endgame for polio eradication, OPV will be replaced with IPV and C-OPV distribution will stop.29 In such a situation, early MV may again be very beneficial in its own right.
Most women are now protected against measles by childhood vaccination rather than natural infection, and this means that MatAb levels have declined dramatically.23 We have previously shown that MV at 4 months elicited protective antibody to nearly all children.26 Hence, from a measles-specific perspective, earlier MV should be considered. To optimise the beneficial NSEs of early MV, however, negative interactions with Penta and C-OPVs should be controlled.
The studies of maternal priming with measles and BCG open a new field of interventions. Earlier vaccination with live vaccines, when maternal antibody is still present, is likely to have beneficial effects on overall child survival. Furthermore, it might be worthwhile to test whether child survival would be improved if a booster dose of MV was given to women of fertile age.
Contributors
The study was planned and initiated by CSB, ABF, SBS, CM, AR, HW, and PA. CSB developed the idea to study the interaction between measles vaccination and maternal antibodies and was the PI for the RCT. SN conducted the final data cleaning and all the statistical analyses. ABF supervised the RCT and organised data collection in connection with the campaigns. CB, IdS and CM conducted the clinical examinations within the trial. AB, ABF, SB, SBS, MBA, NSH, VAD, OB, SMR, LD, SH, OBB, KJJ supervised the demographic data collection, trial enrolment and follow-up for periods of at least 12 months. HCW took part in designing the RCT, GS and FK analysed the blood samples for maternal antibody. The first draft was written by SN, CSB and PA. All authors contributed to the final version. SN, PA and CSB had access to and verify all the study data. SN, PA and CSB were responsible for the decision to submit the manuscript and will act as guarantors of the study.
Data sharing statement
All researchers are encouraged at any time to apply for access to data through the corresponding author and any reasonable use will be approved. Results are shared with the Guinean health authorities. It is up to them to decide whether and how to disseminate results locally.
The lead author (the manuscript's guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Funding
ERC, Danish National Research Foundation, the Danish Council for Development Research, Ministry of Foreign Affairs, Novo Nordisk Foundation, European Union FP7, the Lundbeck Foundation.
Declaration of interests
OB received a scholarship from the Lundbeck Foundation. All other authors declare no competing interests.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101467.
Appendix. Supplementary materials
References
- 1.The Kasongo Project Team Influence of measles vaccination on survival pattern of 7-35-month-old children in Kasongo, Zaire. Lancet. 1981;317(8223):764–767. [PubMed] [Google Scholar]
- 2.Aaby P., Bukh J., Lisse I.M., Smits A.J. Measles vaccination and reduction in child mortality: a community study from GuineaBissau. J Infect. 1984;8:1321. doi: 10.1016/s0163-4453(84)93192-x. [DOI] [PubMed] [Google Scholar]
- 3.Aaby P., Bhuyia A., Nahar L., Knudsen K., de Francisco A., Strong M. The survival benefit of measles immunization may not be explained entirely by the prevention of measles disease: a community study from rural Bangladesh. Int J Epidemiol. 2003;32:106–115. doi: 10.1093/ije/dyg005. [DOI] [PubMed] [Google Scholar]
- 4.Aaby P., Samb B., Simondon F., Coll Seck A.M., Knudsen K., Whittle H. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. Br Med J. 1995;311:481–485. doi: 10.1136/bmj.311.7003.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aaby P., Garly M.L., Balé C., et al. Survival of previously measles-vaccinated and measles-unvaccinated children in an emergency situation: an unplanned study. Pediatr Infect Dis J. 2003;22:798–803. doi: 10.1097/01.inf.0000083821.33187.b5. [DOI] [PubMed] [Google Scholar]
- 6.Aaby P., Garly M.L., Nielsen J., et al. Increased female-male mortality ratio associated with inactivated polio and diphtheria-tetanus-pertussis vaccines: observations from vaccination trials in Guinea-Bissau. Pediatr Infect Dis J. 2007;26:247–252. doi: 10.1097/01.inf.0000256735.05098.01. [DOI] [PubMed] [Google Scholar]
- 7.Aaby P., Jensen H., Samb B., et al. Differences in female-male mortality after high-titre measles vaccine and association with subsequent vaccination with diphtheria-tetanus-pertussis and inactivated poliovirus: re-analysis of West African studies. Lancet. 2003;361:2183–2188. doi: 10.1016/S0140-6736(03)13771-3. [DOI] [PubMed] [Google Scholar]
- 8.Aaby P., Martins C.L., Garly M.L., et al. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial. BMJ. 2010;341:c6495. doi: 10.1136/bmj.c6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aaby P., Martins C.L., Garly M.L., et al. Measles vaccination in the presence or absence of maternal measles antibody: impact on child survival. Clin Infect Dis. 2014;59:484–492. doi: 10.1093/cid/ciu354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Measles vaccines: WHO position paper – April 2017. Weekly Epid Rec. 2017;92:205–228. [Google Scholar]
- 11.Aaby P., Martins C.L., Ravn H., Rodrigues A., Whittle H.C., Benn C.S. Is early measles vaccination better than later measles vaccination? Trans R Soc Trop Med Hyg. 2015;109:16–28. doi: 10.1093/trstmh/tru174. [DOI] [PubMed] [Google Scholar]
- 12.Benn C.S., Fisker A.B., Rieckmann A., Sørup S., Vaccinology Aaby P. Time to change paradigm? Lancet Inf Dis. 2020;20:e274–e283. doi: 10.1016/S1473-3099(19)30742-X. [DOI] [PubMed] [Google Scholar]
- 13.Olesen J.S., Jespersen S., da Silva Z.J., et al. HIV-2 continues to decrease, whereas HIV-1 is stabilizing in Guinea-Bissau. AIDS. 2018;32(9):1193–1198. doi: 10.1097/QAD.0000000000001827. [DOI] [PubMed] [Google Scholar]
- 14.Byberg S., Benn C.S. Placebo use in vaccine trials: caution when using active vaccines as placebo. Vaccine. 2017 Mar 1;35(9):1211. doi: 10.1016/j.vaccine.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 15.http://www.indepth-network.org/resources/indepth-standardized-verbal-autopsy-questionnaire. Accessed 11 March 2022.
- 16.Smits G., Benn C.S., Whittle H., van Binnendijk R., Aaby P., van der Klis F. Maternal measles antibodies and their influence on all-cause mortality following measles vaccination: an alternative to measure very low maternal antibody levels. Clin Infect Dis. 2019;68(10):1758–1760. doi: 10.1093/cid/ciy900. [DOI] [PubMed] [Google Scholar]
- 17.Aaby P., Andersen A., Martins C.L., et al. Does oral polio vaccine have non-specific effects on all-cause mortality? Natural experiments within a randomised controlled trial of early measles vaccine. BMJ Open. 2016;6(12) doi: 10.1136/bmjopen-2016-013335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen A., Fisker A.B., Nielsen S., Rodrigues A., Benn C.S., Aaby P. National immunization campaigns with oral polio vaccine may reduce all-cause mortality: an analysis of 13 years of demographic surveillance data from an urban african area. Clin Infect Dis. 2021;72(10):e596–e603. doi: 10.1093/cid/ciaa1351. [DOI] [PubMed] [Google Scholar]
- 19.Benn C.S., Martins C.L., Andersen A., Fisker A.B., Whittle H.C., Aaby P. Measles vaccination in the presence of measles antibody may enhance child survival. Front Pediatr. 2020;8:20. doi: 10.3389/fped.2020.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen A., Fisker A.B., Rodrigues A., Martins C., Ravn H., Lund N., Biering-Sørensen S., Benn C.S., Aaby P. National immunization campaigns with oral polio vaccine reduce all-cause mortality: a natural experiment within seven randomized trials. Front Public Health. 2018;6:13. doi: 10.3389/fpubh.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleinnijenhuis J., Quintin J., Preijers F., et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benn C.S., Netea M.G., Selin L.K., Aaby P. A small jab – a big effect: non-specific immunomodulation by vaccines. Trends Immunol. 2013;34:431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Fisker A.B., Nebie E., Schoeps A., et al. A two-centre randomised trial of an additional early dose of measles vaccine: effects on mortality and measles antibody levels. Clin Infect Dis. 2018;66:1573–1580. doi: 10.1093/cid/cix1033. [DOI] [PubMed] [Google Scholar]
- 24.Berendsen M., Øland C.B., Bles P., et al. Maternal priming: bCG-scarring in the mother enhances the survival of children with a BCG-scar. J Pediatric Infect Dis Soc. 2020;9(2):166–172. doi: 10.1093/jpids/piy142. [DOI] [PubMed] [Google Scholar]
- 25.Benn C.S., Fisker A.B., Whittle H.C., Aaby P. Revaccination with live attenuated vaccines confer additional beneficial nonspecific effects on overall survival: a review. EBioMedicine. 2016;10:312–317. doi: 10.1016/j.ebiom.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins C., Garly M.L., Bale C., et al. Measles virus antibody responses in children randomly assigned to receive standard-titre Edmonston-Zagreb measles vaccine at 4.5 and 9 months of age, 9 months of age, or 9 and 18 months of age. JID. 2014;210:693–700. doi: 10.1093/infdis/jiu117. [DOI] [PubMed] [Google Scholar]
- 27.Martins C.L., Garly M.L., Balé C., et al. Protective efficacy of standard Edmonston-Zagreb measles vaccination in infants aged 4.5 months: interim analysis of a randomised clinical trial. BMJ. 2008;337:a661. doi: 10.1136/bmj.a661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aaby P., Simondon F., Samb B., et al. Low mortality after mild measles infection compared to uninfected children in rural West Africa. Vaccine. 2002;21:120–126. doi: 10.1016/s0264-410x(02)00430-9. [DOI] [PubMed] [Google Scholar]
- 29.Aaby P., Benn C.S. Beneficial non-specific effects of oral polio vaccine (OPV): implications for the cessation of OPV? Clin Infect Dis. 2017;65:420–421. doi: 10.1093/cid/cix340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.