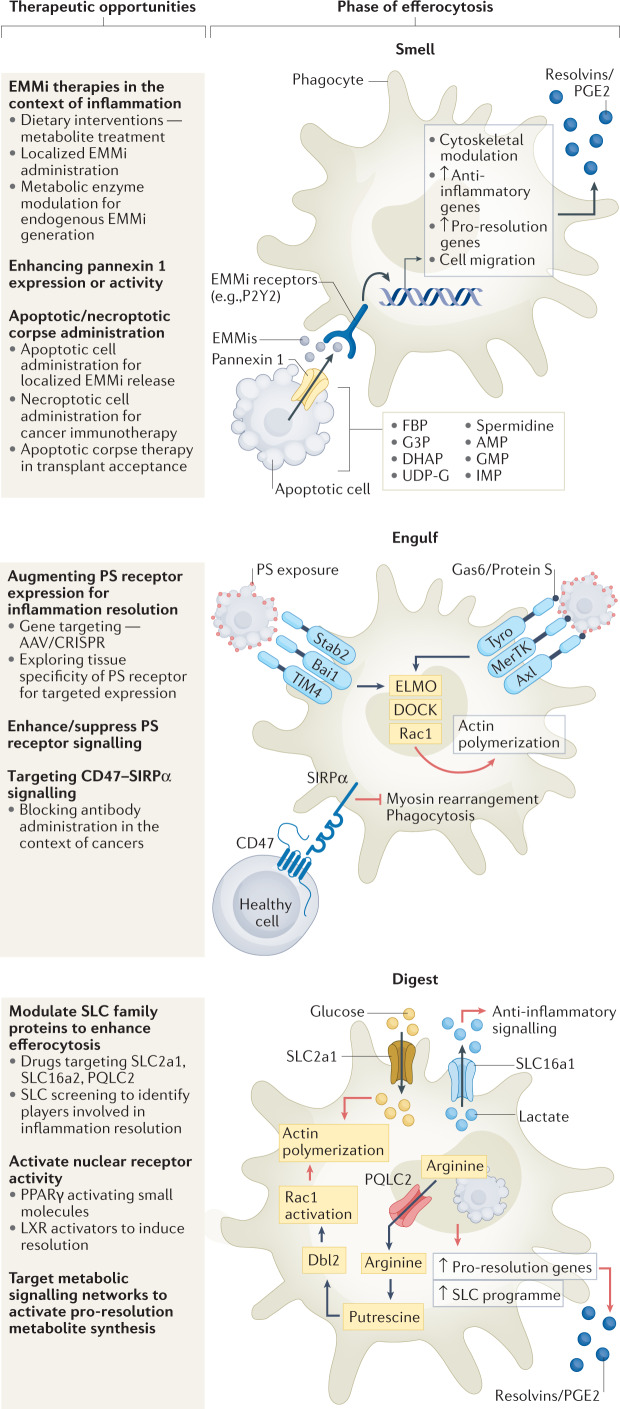
Fig. 1. Steps in efferocytosis and therapeutic opportunities.
Smell phase: clearance of apoptotic cells is a multistep process, initiated by secreted molecules released by the apoptotic corpse, inducing mobilization and migration of phagocytes towards dying cells. Pannexin 1 channel-mediated release of endogenous metabolic modulators (EMMis) from apoptotic cells induces pro-resolution and anti-inflammatory gene expression in phagocytes. Plausible methods for targeting this phase include administration of EMMis, altering activity of metabolic enzymes involved in generation of EMMi or enhancing pannexin 1 channel activity. Administration of donor apoptotic cells, for example via extracorporeal photopheresis (ECP), can reduce transplant rejection in patients and intravenous injections of apoptotic cells suppress anti-donor humoral immune response. In contrast, necroptotic cells elicit a strong immunogenic response and are used in targeting tumour cells. Engulfing phase: apoptotic cell phosphatidylserine (PS) recognition by phagocytes occurs either directly (for example, brain-specific angiogenesis inhibitor 1 (BAI1), T cell immunoglobulin mucin receptor TIM1) or by indirect PS recognition (for example, AXL, MerTK). Key downstream efferocytic signalling involves GTPase RAC1, which is activated via ELMO and DOCK proteins to enhance actin remodelling and formation of the phagocytic cup. Context-dependent modulation of PS receptor expression using gene editing technologies such as CRISPR and AAV-mediated gene delivery helps in disease models. Small-molecule inhibitors of PS receptor signalling are in advanced stages of clinical trials as cancer therapeutics (Table 1). CD47, the classical ‘do not eat-me’ signal, is often overexpressed in pathologies with defective apoptotic cell clearance and blocking CD47–SIRP1 interaction used in cancer treatments and atherosclerosis. Digestion phase: engulfment of multiple apoptotic cells leads to high metabolic burden on phagocytes. Complex signalling machinery enabling cargo digestion and successive cargo engulfment is being deciphered. Nuclear receptors such as PPARγ and liver-X receptor (LXR) enable cargo digestion. Metabolite transfer between intracellular compartments and from extracellular space during efferocytosis (via solute carriers (SLCs)) can improve anti-inflammatory gene expression and continuous efferocytosis66. Further, specialized pro-resolving mediator (SPM) synthesis and secretion by phagocytes is another step in modulating anti-inflammatory function of efferocytosis. AMP, adenosine-5′-monophosphate; Dbl2, guanine nucleotide exchange factor (gene name mcf2); DHAP, dihydroxyacetone phosphate; FBP, fructose-1,6-bisphosphate; G3P, glycerol-3-phosphate; GMP, guanosine-5′-monophosphate; IMP, inosine-5′-monophosphate; PQLC2 (also known as SLC66A1), PQ loop repeat-containing protein 2; UDP-G, UDP-glucose.