Abstract
CRISPR/Cas9 technologies are important tools for the development of gene-drive systems to modify mosquito vector populations to control the transmission of pathogens that cause diseases such as malaria. However, one of the challenges for current Cas9-based drive systems is their ability to produce drive-resistant alleles resulting from insertions and deletions (indels) caused principally by nonhomologous end-joining following chromosome cleavage. Rapid increases in the frequency of such alleles may impair gene-drive dynamics. We explored the generation of indels in the germline and somatic cells in female gene-drive lineages using a series of selective crosses between a gene-drive line, AgNosCd-1, and wild-type mosquitoes. We find that potential drive-resistant mutant alleles are generated largely during embryonic development, most likely caused by deposition of the Cas9 endonuclease and guide RNAs in oocytes and resulting embryos by homozygous and hemizygous gene-drive mothers.
Keywords: NHEJ, indels, gene drive, CRISPR-Cas9, resistance, mutation, mosquitoes
Introduction
CRISPR/Cas9-based gene drives are promising and powerful novel tools for controlling vector-borne diseases due to their ability to bias the inheritance of beneficial synthetic genes and preferred alleles during vector insect reproduction (Deredec et al. 2008; Champer et al. 2016; Gantz and Bier 2016; Godfray et al. 2017). Two approaches currently take advantage of gene-drive systems: population modification and population suppression (Gantz et al. 2015; Hammond et al. 2016; Kyrou et al. 2018; Adolfi et al. 2020; Carballar-Lejarazú et al. 2020; Simoni et al. 2020). Proofs-of-principle for both approaches have been demonstrated in malaria-vectoring mosquito species, and they have high-efficiency drive and are able to spread genes-of-interest into caged laboratory populations in as few as 4 generations when releases of gene drive-carrying males were performed at ratios of 1:1 with wild-type competitors (Kyrou et al. 2018; Adolfi et al. 2020; Carballar-Lejarazú et al. 2020; Simoni et al. 2020). One potential challenge to these gene-drive systems is the generation of guide RNA (gRNA) target-site mutant alleles. There are 2 potential pathways that can create mutant alleles in a Cas9-based gene-drive system (Fig. 1). Mutant alleles can be formed in the germline of an individual carrying 1 copy (hemizygous for the insertion) of the drive system if Cas9/RNA-mediated DNA cleavage does not result in homology directed repair (HDR) (and drive) and a nonhomologous end-joining (NHEJ) event occurs during repair of the lesion. Mutant alleles also can occur due to Cas9/gRNA activity if the complexes are in cells other than the developing germ-line. These somatic Cas9/gRNA complexes may be present if the activity of the promoter driving Cas9 expression is not restricted fully to germline tissues, or could result from maternally-deposited complexes in the oocyte and embryo that cleave the paternally-donated wild-type allele (Gantz et al. 2015; Champer et al. 2017). This somatic activity can result in a mosaic eye-color phenotype comprising wild-type and mutant ommatidia (Gantz et al. 2015; Carballar-Lejarazú et al. 2020). The NHEJ mutant alleles created from these pathways may be resistant to subsequent gRNA-directed Cas9 cleavage and thereby reduce or inhibit introduction of the desired genetic traits if the mutant alleles are passed down to future generations (Hammond et al. 2017; Pham et al. 2019).
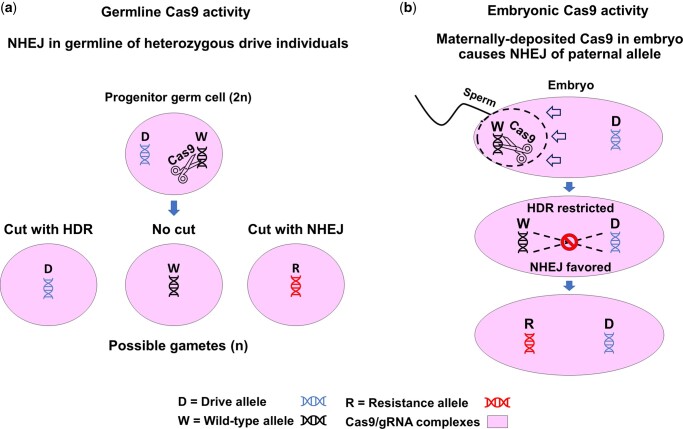
Fig. 1.
Pathways for heritable resistance allele generation. Mutant alleles that confer heritable resistance to Cas9 cleavage can result from activity of the Cas9/gRNA complexes in the progenitor germ cells and the embryos derived from eggs produced by drive-carrying females. a) The Cas9 enzyme (scissors) cleaves DNA in both male and female germline cells during the formation of gametes. Cleavage of a chromosome in a diploid progenitor germ cell prior to meiosis allows homology-directed repair (HDR) that results in conversion of a cell hemizygous for the gene-drive system to homozygosity and results in the biased inheritance of the drive system. The Cas9 enzyme may not cleave the DNA resulting in an un-altered wild-type allele or the Cas9 enzyme may cleave and the DNA repair via nonhomologous end joining (NHEJ) resulting in a potentially drive-resistant allele. Cas9 DNA cleavage in postmeiotic germ cells also would result in NHEJ as there would be no template for HDR. b) Cas9/gRNA complexes may be deposited in the female germ cells and as a result, be present in the resulting embryo following fertilization. These maternally-inherited complexes in the embryo can act on the incoming paternally- donated allele following its release from the sperm head. If the paternally-donated allele is cleaved just after release of the male pronucleus, the physical distance between the paternal and maternal chromosomes within the embryo may prevent the female chromosome serving as a template for HDR resulting in NHEJ and a potentially drive-resistant allele. Furthermore, perdurance of the Cas9/gRNA complexes in somatic cells may produce mutations that lead to mosaicism following blastoderm formation and the observed mosaic “tear” eye phenotype.
We designed and generated a gene-drive line, AgNosCd-1, for population modification of the African malaria mosquito, Anopheles gambiae, that has a Cas9/gRNA-based autonomous gene-drive system marked with the cyan fluorescent protein (CFP). The drive system targets the second chromosome, autosomal cardinal (cd) gene ortholog (Agcd, AGAP003502; VectorBase; Supplementary Table 1) producing a recessive red-eye phenotype in individuals that have both copies of the gene disrupted (Carballar-Lejarazú et al. 2020). AgNosCd-1 achieves drive efficiencies of 98–100% in males and females with a small proportion of the progeny from females exhibiting mosaic eyes (<9%) or loss-of-function phenotypes (<2%) (Carballar-Lejarazú et al. 2020). While the results of small cage trial experiments showed that AgNosCd-1 was not inhibited by the accumulation of drive-resistant alleles, further evaluation of resistant allele formation and impact on gene-drive efficiency is needed to enhance modeling and predictions in anticipation of field-release studies. We examined the generation, inheritance patterns, sequence diversity, and stability of potential drive-resistant gRNA target-site alleles during crosses of AgNosCd-1 individuals to wild-type and mutant cd mosquitoes.
Materials and methods
Mosquito strains and maintenance
Colonies of the An. gambiae G3 strain (referred hereafter as wild-type, WT), the An. gambiae G3 X1 strain (referred hereafter as X1 wild-type, X1-WT) (Volohonsky et al. 2015), the transgenic lines AgNosCd-1 (Carballar-Lejarazú et al. 2020) and AgTP13 (Carballar-Lejarazú et al. in preparation) expressing CFP in the eyes, and 2 strains, AgcdΔ11,14 and AgcdΔ11, with potential loss-of-function mutations in the Agcd gene ortholog (abbreviated hereafter as cd), are the sources of all insects used in this experiment. The AgTP13 line couples the AgNosCd-1 gene-drive system (carrying the 3xP3 promoter driving the CFP as a dominant marker) to a pair of engineered genes encoding the mosquito codon bias-optimized murine single-chain antibodies (scFvs), m1C3 and m2A10, targeting Plasmodium falciparum ookinetes and sporozoites, respectively (Isaacs et al. 2011). A homozygous cd mutant line, AgcdΔ11,14, was established by outcrossing a male mosquito with the cd red-eye phenotype and no CFP fluorescence (cd-/CFP−) recovered from an AgNosCd-1 cage trial (Carballar-Lejarazú et al. 2020) to 15 hemizygous AgNosCd-1 females. The cd-/CFP+ progeny were subsequently intercrossed to generate homozygous cd-/CFP− mosquitoes in the next generation. Molecular analysis of the target site and sequencing detected 2 deletions of 11 (cdΔ11) or 14 (cdΔ14) nucleotides (nt) in length. Subsequent cd-/CFP− intercrosses and molecular screening generated the homozygous AgcdΔ11 line, confirming that this deletion alone imposes a loss-of-function phenotype.
All mosquitoes were maintained at 27°C with 77% humidity and a 12-h day/night, 30 min dusk/dawn lighting cycle. Adult females were provided rabbit blood (Colorado Serum Company, CO) through artificial membranes (Hemotek, Inc., Blackburn, UK).
Mosquito mating and screening
Mosquitoes were mated in pools with the number of males and females in each pool determined by their availability as the number of each unique phenotype generated by 1 cross and used in a subsequent cross could not be predetermined. Mating events of ∼100 individuals were performed in 83-ounce (2455 cm3) cages and matings of <100 individuals in 46-ounce (1360 cm3) cages. Males and females in each cross were allowed to mate for 5 days, then provided a blood meal and oviposition cup 3 days post blood meal. The progeny of each cross were screened using light microscopy and UV-fluorescence for eye phenotypes (eye color: red, cd mutant; black, wild-type) and the presence or absence of CFP fluorescence, and hand-sorted as pupae by sex. Pupae were kept separately in 16-ounce (473 cm3) cups by sex and eye phenotypes and subsequent emerging adults were maintained as virgins. Individuals of each unique phenotype were used either in subsequent mating events or preserved at −20°C for molecular analyses.
Crosses to generate potential drive-resistant alleles
Maternal deposition of Cas9 and gRNAs into eggs can cause mutations following Cas9-induced, nonhomologous end-joining (NHEJ) resulting in indel mutations at the gRNA target site of the male-contributed allele (Gantz et al. 2015). We outcrossed separately 50 hemizygous or homozygous AgNosCd-1 females to 50 WT males in triplicate and screened the progeny for the red-eye phenotype that would indicate that an incoming wild-type cd allele from the males had been mutated. We refer to mosquitoes as hemizygous or homozygous based on whether they carry 1 or 2 copies of the drive system, respectively.
Crosses to assess mutant cd allele inheritance
Mutant cd allele/AgNosCd-1 hemizygote intercrosses
A total of 47 progeny with a red-eye color and CFP+ phenotype generated from a homozygous AgNosCd-1 female outcrossed to WT males were presumed to have 1 copy of the drive allele and 1 copy of a nonfunctional mutant cd allele or some level of mosaicism not apparent in the eye for indel and wild-type alleles. All individuals with this phenotype were combined and allowed to intercross and the progeny screened again for red-eyes and CFP fluorescence. The red-eye/CFP− mosquitoes were presumed to have 1 (homozygous) or 2 heteroallelic nonfunctional mutations in the cd gene.
Mutant cd allele/AgNosCd-1 hemizygote outcrosses
A total of 15 red-eye/CFP+ hemizygous males were removed from the previous hemizygote intercross cages after their mating period, allocated to a new cage and outcrossed to 75 WT females. All progeny were screened for the red-eye phenotype and CFP fluorescence.
Mutant cd allele homozygote intercross
Approximately 60 mixed-sex progeny generated from the previous hemizygote intercross with evidence of 2 copies of nonfunctional mutant cd alleles based on the red-eye, CFP− phenotype were combined and allowed to intercross. All progeny were screened for the red-eye and CFP fluorescence phenotypes.
Molecular analysis of individual mosquitoes from crosses
Genomic DNA was extracted from individual mosquitoes using either a Promega Wizard Genomic DNA Purification Kit or Extract-N-Amp PCR kit (Sigma). Two oligonucleotide primers (5′GTACTCGTACGGTCGCTCCTTA3′ and 5′ATTGTTGTTGCAGATGAGTCGT3′) were designed to amplify a DNA fragment of 396 base pairs (bp) in length encompassing the gRNA-directed Cas9 cleavage site. Two additional primers (5′CTCCAGCAGCTCCTTATCGC3′ and 5′GCTTGTTTGAATTGAATTGTCGC3′) were used to amplify a 742 bp DNA fragment spanning the cd gene left homology arm and the drive cassette to verify the presence of the drive allele. Sanger sequencing was used to analyze potential indels in genomic DNA from mosquitoes recovered from each mating event (Genewiz, USA).
Mutant cd allele dynamics in a gene-drive population
An 83-ounce (2455 cm3) cage was set up with 50 red-eye/CFP− males from the cd homozygous mutant line Ag(cd Δ11,14) containing a mixture of mutant cd alleles, cdΔ11 or cdΔ14, and 50 AgTP13 gene-drive homozygous females. Progeny were screened for eye color and CFP fluorescence, and 300 pupae were selected randomly to populate the next generation. The population was maintained in this manner for 8 generations to assess the stability over time of mutant cd and drive alleles in a mixed population.
cd Δ11 allele dynamics in wild-type populations
Triplicate 83-ounce (2455 cm3) cages were established with 150 homozygous AgcdΔ11 males, which contained only the 11 nucleotide deletion, and 150 X1-WT females. All progeny were screened for eye color and fluorescence phenotypes, 300 pupae selected randomly and transferred into a new cage for the next generation. The cage trials were maintained for 10 generations to determine the dynamics of the cdΔ11 alleles in the population over time.
Results
Assessment of NHEJ-induced indel allele formation in embryos from AgNosCd-1 females
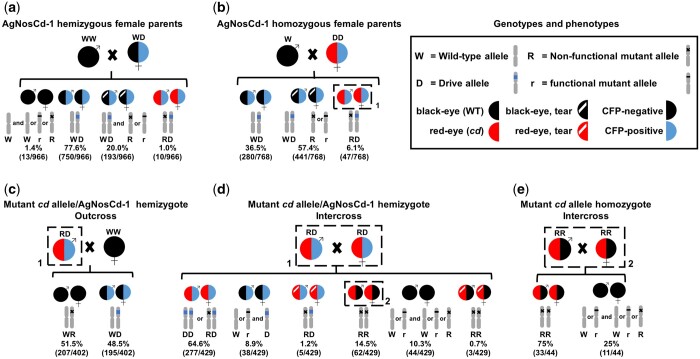
Separate outcrosses of homozygous and hemizygous AgNosCd-1 females to WT males were used to determine the frequencies of NHEJ-induced indel allele formation due to Cas9 deposition in embryos (Fig. 2, a and b). Next-generation progeny with a red-eye, CFP positive (cd-/CFP+) phenotype were likely to have a nonfunctional indel allele formed during early embryonic development. These individuals would have the drive allele (CFP+) inherited from their mothers and 1 mutant cd allele resulting from mutation of the paternally derived gene. The frequency of recovery of red-eye phenotypes was significantly higher in progeny resulting from outcrosses of homozygous AgNosCd-1 females (6.1% red-eye/CFP+) compared to hemizygous AgNosCd-1 outcrosses (1.0% red-eye/CFP+) (2 proportion z-test, z = 5.94; P ≤ 0.00001) (Fig. 2, a and b; Supplementary Tables 2 and 3). Sequencing around the gRNA target site of samples of cd-/CFP+ progeny from both homozygous and hemizygous AgNosCd-1 females (18 and 4 individuals, respectively) identified 29 distinct indels, with 1 mutation appearing in individuals (10 and 20, Supplementary Table 4) recovered from both crosses. These data support the conclusion that the indels occurred in the fertilized embryo as they all were different, which would not be expected if the progeny shared a mutant allele inherited from either parent. Eight red-eye/CFP+ individuals (2, 4, 8, 9, 14, 17, 19, and 22) had 2 different mutant cd alleles along with the drive allele, indicating that multiple indels were generated during embryonic development resulting in a mosaic mosquito. Six indels (7, 9b, 12, 13, 14a, and 17b) were in frame, but the size or position of the indels affected the function of the resulting product and failed to restore the wild-type phenotype. We also observed a greater portion of phenotypically mosaic individuals (termed “tear eye”; Carballar-Lejarazú et al. 2020) in the progeny of homozygous AgNosCd-1 outcrosses (57.4% tear-eye/CFP+) compared to hemizygous AgNosCd-1 outcrosses (20.0% tear-eye/CFP+) (z = 16.06; P < 0.00001) (Fig. 2, a and b). The high frequency of recovery of mosaic, tear-eye phenotype mosquitoes is consistent with most embryonic Cas9 cleavage of the incoming wild-type male cd allele occurring later in development, after several mitotic divisions, which gives rise to mutant alleles in some, but not all, somatic cells (Carballar-Lejarazú et al. 2020, Fig. 1).
Fig. 2.
Crosses to generate potential drive-resistant cd mutant alleles. Crosses were made with homo- or hemizygous AgNosCd-1 (CFP+) males and females to wild-type (outcrosses) or sibling (intercrosses) mosquitoes to detect and monitor the formation and inheritance patterns of mutant cd alleles. Graphic representations of parental and progeny phenotypes (half-circle coloration) and alternative genotypes (marked chromosome symbols) are presented along with phenotypic frequencies. The left half of each circle denotes the eye color phenotype of wild-type (black), cd mutant (red) or mosaic, tear (white slash) in a wild type- or cd-mutant background (black or red, respectively). The right half of the circle denotes negative or positive for CFP fluorescence (black or blue, respectively). The numbers of each phenotype seen and total number of mosquitoes scored are shown in parentheses. Top panels: crosses used to generate mutant cd alleles, a) hemizygous and b) homozygous female CFP+ individuals were used to increase the likelihood of recovery of NHEJ-induced mutant cd alleles resulting from maternal effects as seen in Carballar-Lejarazú et al. (2020), with 50 AgNosCd-1 (hemizygous or homozygous) females crossed with 50 wild-type (WT) males in triplicate. Individual experimental details are presented in Supplementary Tables 2 and 3. Bottom panels c and d: crosses with selected progeny obtained in b (dashed box outline labeled “1”) with potential mutant cd alleles to assess the inheritance patterns of mutant cd alleles in the presence or absence of a chromosome carrying the drive system. c) A total of 15 red-eye/CFP+ hemizygous males were removed from the hemizygote intercross cage (Cross D) after their mating period, allocated to a new cage and outcrossed to 75 WT females. d) A total of 47 female and male red-eye/CFP+ homozygotes generated in b were intercrossed. e) Phenotypes and possible genotypes of progeny from an intercross of ∼60 mixed-sex red-eye/CFP− progeny from d (dashed box outline labeled “2”).
Assessment of functional mutant cd allele formation in AgNosCd-1 female germlines
Progeny negative for the gene-drive allele with a wild-type, black eye color (black-eye/CFP−) resulting from outcrosses of hemizygous AgNosCd-1 females to WT males may have different types of allele combinations. They may have 2 wild-type cd alleles, one from their father and the second as a result of unsuccessful germline drive conversion in their hemizygous mothers. They may have 1 paternally-inherited wild-type allele and a maternally-inherited mutant cd allele due to NHEJ following unsuccessful germline drive conversion. They also may have a wild-type allele from the mother due to lack of conversion and a functional or nonfunctional allele from the male cd locus mutated by the maternal deposition of Cas9 in the embryo. There were no black-eye/CFP− progeny recovered from outcrosses of homozygous AgNosCd-1 females to wild-type males because both of the parental female cd genes have insertions of the gene-drive construct (Fig. 2b). However, 1.4% (13/966) black-eye/CFP− progeny were recovered from outcrosses of hemizygous AgNosCd-1 females to wild-type males (Fig. 2a). Molecular analysis was done to examine if these individuals were homozygous or heterozygous for wild-type alleles to estimate the rate of formation of resistance allele in the germline. All 13 black-eye/CFP− individuals were homozygous for WT alleles indicating that no functional mutant alleles were generated in the AgNosCd-1 hemizygous female germline with the sample size used here (Supplementary Table 5).
Inheritance of nonfunctional mutant cd alleles
Mutant cd allele-bearing individuals were mated to assess how the alleles are inherited in the presence or absence of gene-drive alleles. Two crosses with mutant cd allele and drive allele hemizygotes, red-eye/CFP+, and 1 cross with cd mutant allele homozygotes, red-eye/CFP−, were performed.
Mutant cd allele/AgNosCd-1 hemizygote outcrosses
Males hemizygous for mutant cd (red-eye) and gene-drive (CFP+) alleles were outcrossed to WT females to determine whether the drive allele could convert cd mutant alleles in the male germline (Fig. 2c). The mutant cd alleles represent a number of different genotypes in individual male progeny resulting from the cross depicted in Fig. 2b. Segregation of the gene-drive allele was consistent with Mendelian inheritance with the CFP+ and CFP− phenotypes seen in 48.5% (195 of 402) and 51.5% (207 of 402), respectively (Χ2 = 0.179, P = 0.67), of the progeny. This is consistent with no gene-drive conversion in the germline of the AgNosCd-1 hemizygote males, and we interpret the mutant target cd alleles to be drive-resistant.
Mutant cd allele/AgNosCD-1 hemizygote intercrosses
Intercrosses of hemizygous gene-drive and mutant cd males and females were done and the resulting CFP+:CFP− phenotypic ratio in the progeny was 74.6% (320/429):25.4% (109/429), consistent with a canonical 3:1 Mendelian phenotypic inheritance pattern (Χ2 = 0.038, P = 0.845; Fig. 2d). Again, these data support the interpretation that the mutant cd target alleles are resistant to the gene-drive system.
The distribution of eye-color phenotypes among the progeny of the hemizygous intercross reflects the impact of the maternal deposition of the Cas9 protein during early development. While this was an intercross of parents hemizygous for the gene-drive construct as evidenced by all parents being CFP+, the other allele was expected to be a nonfunctional mutant cd allele as all parents also had the red-eye color phenotype. However, 6 eye-color phenotypes were recovered: red-eye/CFP+, red-eye/CFP−, black-eye/CFP+, black-eye/CFP−, mosaic “tear”/CFP+ and mosaic “tear”/CFP− (Fig. 2d). In addition, a rare mosaic phenotype in an apparent mutant cd, red-eye background was seen, most likely reflecting a heteroallelic combination of a null and hypomorph mutation (Supplementary Fig. 1). Molecular analyses showed that the genotypes of these individuals can explain the observed phenotypes and provided more insight into the diversity of drive-resistant, mutant cd alleles created in AgNosCd-1 female-derived embryos (Supplementary Table 6). The 10 red-eye/CFP+ progeny were either homozygous for the drive alleles (6) or hemizygous with 1 drive allele and 1 out-of-frame mutant allele (4). This is expected based on the assumption that their hemizygous parents contributed either an AgNosCd-1 allele or a nonfunctional mutant cd allele. The 9 black-eye/CFP+ phenotypic individuals were not expected because the red-eye hemizygous parents should not have been able to provide a functional cardinal gene. However, the genotypes of these progeny revealed them to be hemizygous for the drive allele and 1 of 2 versions of a functional resistant allele with small (6 nt) in-frame mutations. The 12 black-eye/CFP− individuals each had 1 of 6 heteroallelic genotypes characterized by 1 out-of-frame and 1 small in-frame mutant cd allele. The 10 red-eye/CFP− individuals were an expected phenotype but they had an unexpected diversity of alleles with individuals with either 2 out-of-frame mutant cd alleles or 1 out-of-frame allele along with an allele with large in-frame mutation resulting from 5 heteroallelic genotypes and 10 total alternate alleles.
Comparisons of the DNA sequences of the alleles in the black-eye progeny (Supplementary Table 6, derived from the cross depicted in Fig. 2d) and a subset of their mutant cd red-eye/CFP+ parents (Sequences 1–18, Supplementary Table 4) support the conclusion that some of the parents were genotypically mosaic. The black-eyed progeny likely resulted from mutant red-eye/CFP+ parents that carried 2 different cd mutant alleles in their germline along with the drive allele. The in-frame mutant cd alleles were transmitted subsequently to the progeny and restored the wild-type, black-eye phenotype. The mutant red-eye color originates in a clone or clones of cells that formed the ommatidia and had nonfunctional cd mutant alleles. The recovery at low frequency of the mosaic “tear”-eye phenotypes is consistent with this interpretation (Fig. 2d).
Mutant cd allele homozygous intercrosses
An intercross of the small number (14) of red-eye/CFP− (drive system-negative) mosquitoes originating from the hemizygous intercross (Fig. 2d) was made to further understand the inheritance of the resistant alleles (Fig. 2e). The expected result of an intercross of red-eye/CFP− individuals, with presumably 2 nonfunctional mutated alleles, is 100% of the offspring with 2 nonfunctional alleles, sharing the red-eye/CFP− phenotype of their parents. However, this intercross again produced an unexpected outcome as offspring with 2 distinct phenotypes, red-eye/CFP− and black-eye/CFP-, were recovered. Sequencing data revealed that the red-eye/CFP− individuals carried as expected 2 different out-of-frame mutated cd alleles (1–5.a , 1–5.b) (Supplementary Table 7). Seven of the 8 black-eye/CFP− individuals (2–8) sequenced were hemizygous with 1 out-of-frame resistant allele and 2 different in-frame resistant alleles (2–8.b and 2–8.c) that had the large insertion fragment coming from a region downstream of the target site, resulting in duplication of part of the gene that restored function. One individual had both an in-frame and out-of-frame allele (1.a, 1.b).
Calculating the rate of formation of heritable, functional potentially drive-resistant alleles generated by NHEJ is complicated by phenotypic and genotypic mosaicism, cluster effects from events in single germline progenitor cells and the ability to sequence the target sites of large numbers of mosquitoes. The difficulty is demonstrated from the work from the B and D crosses in Fig. 2. The frequency of mosaic, tear eye/CFP+ (57.4%) and red-eye/CFP+ (6.1%) progeny was high (63.5%) from a cross of wild-type males with presumed homozygous gene-drive mothers (red eye/CFP+) indicating a high rate of NHEJ in the embryos of those females (Fig. 2b). Recall that a functional resistance allele would be expected to produce a dominant wild-type (black-eye) phenotype and none of the red-eye/CFP+ have that. However, an intercross of the red-eye/CFP+ progeny from this cross produced 8.9% black-eye/CFP+ and 10.3% black-eye/CFP− progeny for a combined total of 19.2% black-eye mosquitoes, presumably carrying one or more functional NHEJ alleles inherited from their red-eye/CFP+ mothers (Fig. 2d). These females must have been mosaic in their germlines for black-eye conferring alleles. The sequences of the DNA surrounding the target sites of 19 of these mosquitoes revealed 14 distinct NHEJ indel alleles, 8 of which were in-frame as would be needed to provide the black-eye phenotype. All 9 of the 8.9% black-eye/CFP+ progeny were hemizygous for the gene-drive construct and a functional allele, as expected. The 10 black-eye/CFP− progeny showed 2 apparent clusters (identical genotypes, 1–4 and 6–7, Supplementary Table 6). The challenge then becomes trying to define the denominator. We sampled by subsequent mating only a small fraction of the progeny (14 total red eye/CFP+) of the original cross (Fig. 2b) and it can be expected that the whole cohort is likely to have produced more than the 14 distinct alleles. The best way to get an accurate estimate would be to sequence a large number of the progeny of the cross in Fig. 2b and use those results to estimate the frequency.
Population dynamics of the homozygous AgcdΔ11,14 line in cage population competition with a gene drive line
A cage population trial initiated with 50 heteroallelic AgcdΔ11,14 [cd mutant line containing 2 different mutations, an 11-nucleotide-deletion cdΔ11and a 14-nulceotide-deletion, cdΔ14 (Supplementary Table 8)] males and 50 homozygous AgTP13 gene-drive females was performed to evaluate how a potential drive-resistant mutant cd allele might affect gene-drive dynamics over multiple generations. AgTP13 couples the autonomous AgNosCd-1 gene-drive system to 2 antimalarial single-chain antibodies, m1C3 and m2A10, driven by the carboxypeptidase-encoding and vitellogenin-encoding gene promoters, respectively (Carballar-Lejarazú et al. in preparation).
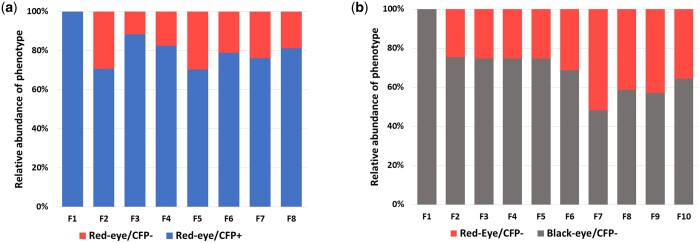
The first generation (F1) progeny are expected to be hemizygous for cdΔ11,14 and AgTP13 drive alleles and therefore be 100% red-eye/CFP+. This expectation was verified in all the F1 progeny (Fig. 3a, Supplementary Table 9). However, a small number of progeny recovered in the first generation had only the cdΔ11 allele being represented in subsequent generations (see Materials and Methods: “Mutant cd allele dynamics in a gene-drive population”). In addition, it was anticipated that cdΔ11, if a true resistance allele, would inhibit AgTP13-mediated target-site conversion in subsequent interbreeding populations (F2–F8) resulting in phenotypic ratios of ∼3:1 CFP+:CFP− as the hemizygous mosquitoes continue to intermate. The percentages of CFP+ drive-carrying mosquitoes fluctuated between 71% and 88%, with the 2 generations (F3, F4) above 82% being statistically significant (Pearson’s Χ2 test with Bonferroni correction, Χ2 ≥ 23.5, P ≤ 8.4 × 10−6). While there is a failure of AgTP13 to achieve full introduction (every mosquito having at least 1 copy of the gene-drive construct), thus verifying that AgcdΔ11 is a true drive-resistant line, there appears to be no major load on the drive allele and it can be maintained in the population even in the presence of the resistance allele. The fluctuations in the inheritance bias may reflect stochastic properties of the cage trial design or possible low-frequency cleavage of the resistant cdΔ11 allele.
Fig. 3.
Population dynamic of mutant, drive resistant line AgcdΔ11,14 in the presence of drive or wild-type (WT) alleles. a) Male homozygous red-eyed AgcdΔ11,14 mutant mosquitoes were crossed with AgTP13 (red-eyed/CFP+) females. b) Triplicate crosses between homozygous red-eyed mutant AgcdΔ11 male and Anopheles gambiae X1 females were performed. Averaged data for each generation are presented here. Individual experimental details are presented in Supplementary Table 10. Progeny from both a and b were screened and the percentages recorded of the eye color (cd, red-eye or WT, black-eye) and fluorescence (CFP+ or CFP−) phenotypes. Progeny were allowed to intercross for subsequent generations.
Population dynamics of the homozygous AgcdΔ11 line in cage population competition with WT
AgcdΔ11males were crossed in the presence of equal numbers of wild-type X1-WT females to assess their competitive success over time at contributing to subsequent generations in a wild-type population. The first generation (F1) progeny of these crosses was heterozygous for 1 wild-type and 1 cdΔ11 allele and show the black-eye phenotype (Fig. 3b, Supplementary Table 10). Assuming that there is no competitive disadvantage associated with the cdΔ11 mutant allele, it is expected that all subsequent generations would maintain an ∼3:1 ratio of wild-type black-eye to cdΔ11 red-eye phenotypes as the populations continue to interbreed. No significant deviations from this ratio were observed for the next 4 (F2–F5) generations (χ2 ≤ 0.054, P > 0.8). However, at the F6 and subsequent generations (F7–F10), the percentages of homozygous cdΔ11 mutant progeny increased and fluctuate between 30% and 50%. This increase and fluctuation varied greatly among the 3 experimental replicates (Supplementary Table 10). These data support the conclusion that the cdΔ11 nonfunctional resistant allele does not carry a greater load than wild-type alleles and the red-eye phenotype is not selected against in a mixed population.
Discussion
AgNosCd-1 hemizygote female lineages were shown previously to have a high drive efficiency with the majority of progeny (∼95%) from AgNosCd-1 female outcrosses to wild-type mosquitoes exhibiting the gene-drive (CFP+) phenotype (Carballar-Lejarazú et al. 2020). This high-drive efficiency was attributed in part to low rates of resistance allele formation as a consequence of NHEJ. However, it also was observed that 2 copies of a Cas9-containing allele can increase the rates of resistance allele formation, presumably due to increased levels of Cas9-mediated cleavage and subsequent NHEJ in embryos (Gantz et al. 2015; Champer et al. 2017, 2018). Although homozygous AgNosCd-1 females had a higher embryonic NHEJ allele generation rate compared to hemizygous AgNosCd-1 (6.1% and 1.4%, respectively), they are lower than what has been observed with similar crosses in other Cas9-based gene-drive systems in Anopheles stephensi (estimated at 65%–90%) or Drosophila melanogaster (estimated between 5% and 100% dependent on drive and genetic background) (Gantz et al. 2015; Champer et al. 2017; 2018; 2019). The lower rate of embryonic NHEJ allele generation might be in part attributed to the differences among drive systems in the control DNA used to express the Cas9 nuclease. AgNosCd-1 utilizes the An. gambiae nanos ortholog regulatory DNA that may better localize Cas9 activity to the germline cells, unlike other promoters such as vasa, which can cause the expression of Cas9 outside of the germline (Terradas et al. 2021).
The progeny of crosses affected by embryonic Cas9 activity produced NHEJ alleles that could inhibit gene-drive. Mutant cdΔ11 alleles appear to not be converted (or converted at a low rate, Fig. 3a) in the germline of mosquitoes hemizygous for the AgNosCd-1 or AgTP13 drive systems, and they suppress the significant biased inheritance in both intercrosses and outcrosses to wild-type mosquitoes.
A significant observation from these crosses and subsequent sequencing of the progeny was the observation that the red-eyed/CFP+ mutant cd allele/AgNosCd-1 hemizygous individuals generated from embryonic Cas9 activity were mosaic. The DNA sequences of mutant cd allele/AgNosCd-1 hemizygous individuals revealed some individuals with 2 different mutant cd alleles in addition to the drive allele. Evidence of mosaicism also was observed in the red-eyed/CFP+ mutant cd allele/AgNosCd-1 hemizygous intercross as offspring with apparent wild-type (black) eyes were observed, despite no black-eye phenotypes in the parents. These phenotypes were a result of hidden functional NHEJ alleles that must have been present in the parental germline but not in the somatic cells that give rise to the eye. Importantly, although the red-eyed/CFP+ mutant cd allele/AgNosCd-1 hemizygous individuals generated as a result of embryonic Cas9 activity were mosaic for multiple resistant alleles, we did not see evidence of true wild-type alleles in these mosaic insects. The reduced bias of the drive alleles in the crosses also supports the conclusion that mosaicism did not likely include any wild-type alleles.
The dynamics of the resistance alleles evaluated here in mixed populations consisting of either gene-drive alleles or wild-type alleles did not give any clear evidence of any fitness cost, benefit, or increased load of the resistance alleles. It was anticipated that a gene-drive allele might carry more of a load compared to a nonfunctional resistance allele due to the potentially negative fitness effect of expression of the gene drive elements, yet it was observed over multiple generations of a mixed drive allele and resistant allele population that both the drive allele and resistance alleles remained near their expected proportions based on Mendelian inheritance.
The creation of the homozygous cd resistant AgcdΔ11 strain allows us to address questions of any effect the mutations in cd alone may have on overall fitness in An. gambiae and impact on gene-drive dynamics. The enzyme, HPX6, encoded by the cd gene (AGAP003502) is annotated in VectorBase as a heme peroxidase and has a catalytic role in the tryptophan metabolism pathway where it is involved in one of the last steps in the formation of eye pigments (Carballar-Lejarazú et al. 2020). Mutations in genes encoding enzymes in the tryptophan metabolic pathway can lead to a number of deleterious pleiotropic effects in insects (Linzen, 1974; Savvateeva-Popova et al. 2003; Osanai-Futahashi et al. 2016; Zhang et al. 2017; Xu et al. 2020; Zhuravlev et al. 2020). For example, mutations in the gene encoding the kynurenine hydroxylase enzyme (also called kynurenine mono-oxygenase) are orthologs of the cinnabar (cn) gene and produce a white-eye phenotype in Aedes aegypti, Culex quinquefasciatus and An. stephensi (Cornel et al. 1997; Han et al. 2003; Gantz et al. 2015; Feng et al. 2021). Female mosquitoes of these species with spontaneous and induced mutations in their respective cn orthologs have impaired survival and reproductive phenotypes that vary in type and severity among the species (Bottino-Rojas et al. 2022).
The red color of the eyes in An. gambiae homozygous mutant cd larval, pupal and newly emerged mosquitoes is an indication of the reduced levels of eye pigments in these insects (Carballar-Lejarazú et al. 2020). However, the fact that they have any color at all likely results from the nonenzymatic conversion of the precursor, 3-hydroxykynureine, into an ommochrome, albeit with a titer that is less than that in wild-type mosquitoes. This interpretation is consistent with the observation that the eye color darkens to near-wild in mutant adults within 2 days after eclosion, presumably as the nonenzymatic conversion continues in this stage. A similar phenotype was observed in a mutant line of Ae. aegypti (Chen et al. 2021). Furthermore, analysis of the accumulation of metabolic products in mosquitoes carrying homozygous gene-drive disruptions of the cd locus showed a significantly higher accumulation of xanthurenic acid than controls, presumably resulting from the efficient enzymatic conversion of 3-hydroxykynurenine (Carballar-Lejarazú et al. 2020).
Molecularly characterized, nonfunctional cd mutations have not been reported in wild-derived An. gambiae. However, a spontaneous mutation, red-eye (r), with a similar phenotype has been described in this species and similar naturally-derived mutations, crimson in Culex pipiens and red-eye (re) in Ae. aegypti have been described (Bhalla 1968; Beard et al. 1995; Rasgon and Scott 2004). Linkage assessments of crimson and re (3L and sex-linked, respectively) are inconsistent with these being true orthologs of the Agcd locus, which was assigned to 2R (VectorBase), although it is likely that synteny for these chromosomes is not conserved among these species. In particular, re linked to first chromosome, was shown to be an Ae. aegypti cd ortholog using CRISPR gene knock-out tools (Chen et al. 2021).
Impacts on fitness from null mutations in the various red-eye phenotype mosquitoes differ among studies. For example, both positive and negative effects have been reported for Ae. aegypti while negative effects only were described in C. pipiens (Lorimer et al. 1976; Wood et al. 1977; Rasgon and Scott 2004). While it is assumed that wild-type individuals are likely to out-compete mutant cd, drive-resistant individuals in a mixed population over multiple generations, our data are consisted with no negative impact on the reproductive success for mutant cd individuals in the laboratory environment where they compete equally with wild-type counterparts. Future semi-field studies will need to be completed in order to determine if cd individuals can compete with wild-type individuals outside of the laboratory where factors such as natural sunlight and larval predation can be mimicked and fitness costs re-assessed. However, it is likely that the strength of the drive bias will compensate for any load imposed by the system.
In summary, we have shown that inheritance through maternal lineages can result in the generation of potentially drive-resistant target sites in the gene-drive mosquitoes tested here. However, these observations do not preclude the use of this or a similar system for further development as the basis of a population modification system for controlling mosquito-borne pathogens.
Data availability
All mosquito strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Supplemental material is available at GENETICS online.
RC-L, TT, TBP, and AAJ designed experiments; RC-L, TT, and TBP conducted experiments; RC-L, TT, TBP, and AAJ analyzed data, and RC-L, TT, TBP, and AAJ prepared the manuscript.
Supplementary Material
Acknowledgments
The authors are grateful to Drusilla Stillinger, Parrish Powell, and Kiona Parker for mosquito husbandry. Rhodell Valdez helped with preparation of the manuscript.
Funding
Funding was provided by the University of California Irvine Malaria Initiative. AAJ is a Donald Bren Professor at the University of California, Irvine.
Conflicts of interest
The authors declare no conflict-of-interests.
Contributor Information
Rebeca Carballar-Lejarazú, Department of Microbiology & Molecular Genetics, University of California, Irvine, Irvine, CA 92697-4025, USA.
Taylor Tushar, Department of Microbiology & Molecular Genetics, University of California, Irvine, Irvine, CA 92697-4025, USA.
Thai Binh Pham, Department of Molecular Biology & Biochemistry, University of California, Irvine, Irvine, CA 92697-3900, USA.
Anthony A James, Department of Microbiology & Molecular Genetics, University of California, Irvine, Irvine, CA 92697-4025, USA; Department of Molecular Biology & Biochemistry, University of California, Irvine, Irvine, CA 92697-3900, USA.
Literature cited
- Adolfi A, Gantz VM, Jasinskiene N, Lee HS, Hwang K, Bulger EA, Ramaiah A, Bennett JB, Terradas G, Emerson JJ, et al. A population modification gene-drive rescue system dominantly eliminates resistance alleles in the malaria mosquito, Anopheles stephensi. Nat Commun. 2020;11:5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla SC. Genetic aspects of pteridines in mosquitoes. Genetics. 1968;58(2):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard CB, Benedict MQ, Primus JP, Finnerty V, Collins FH. Eye pigments in wild-type and eye-color mutant strains of the African malaria vector Anopheles gambiae. J Hered. 1995;86(5):375–380. [DOI] [PubMed] [Google Scholar]
- Bottino-Rojas V, Ferreira I, Nunes RD, Feng X, Pham TB, Kelsey A, Carballar-Lejarazú R, Gantz V, Oliveira PL, James AA. Beyond the eye: kynurenine pathway impairment causes midgut homeostasis dysfunction and survival and reproductive costs in blood-feeding mosquitoes. Insect Biochem Mol Biol. 2022;142:103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballar-Lejarazú R, Ogaugwu C, Tushar T, Kelsey A, Pham TB, Murphy J, Schmidt H, Lee Y, Lanzaro GC, James AA. Next-generation gene drive for population modification of the malaria vector mosquito, Anopheles gambiae. Proc Natl Acad Sci USA. 2020;117(37):22805–22814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballar-Lejarazú R, Dong YM, Pham TB, Tushar T, Marshall J, Dimopoulos G, James AA. Autonomous gene-drive strains for population modification of the African malaria mosquito, Anopheles gambiae. (in preparation).
- Champer J, Buchman A, Akbari OS. Cheating evolution: engineering gene drives to manipulate the fate of wild populations. Nat Rev Genet. 2016;17(3):146–159. [DOI] [PubMed] [Google Scholar]
- Champer J, Reeves R, Oh SY, Liu C, Liu J, Clark AG, Messer PW. Novel CRISPR/Cas9 gene drive constructs reveal insights into mechanisms of resistance allele formation and drive efficiency in genetically diverse populations. PLoS Genet. 2017;13(7):e1006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champer J, Liu J, Oh SY, Reeves R, Luthra A, Oakes N, Clark AG, Messer PW. Reducing resistance allele formation in CRISPR gene drive. Proc Natl Acad Sci USA. 2018;115(21):5522–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champer J, Chung J, Lee YL, Liu C, Yang E, Wen Z, Clark AG, Messer PW. Molecular safeguarding of CRISPR gene drive experiments. eLife. 2019;8:e41439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Compton A, Nikolouli K, Wang A, Aryan A, Sharma A, Qi Y, Delinger C, Hemple M, Augustinos A, et al. Marker-assisted mapping enables effective forward genetic analysis in the arboviral vector Aedes aegypti, a species with vast recombination deserts. bioRxiv. 2021. doi: 10.1101/2021.04.29.442065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornel AJ, Benedict MQ, Rafferty CS, Howells AJ, Collins FH. Transient expression of the Drosophila melanogaster cinnabar gene rescues eye color in the white eye (WE) strain of Aedes aegypti. Insect Biochem Mol Biol. 1997;27(12):993–997. [DOI] [PubMed] [Google Scholar]
- Deredec A, Burt A, Godfray HC. The population genetics of using homing endonuclease genes in vector and pest management. Genetics. 2008;179(4):2013–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, López Del Amo V, Mameli E, Lee M, Bishop AL, Perrimon N, Gantz VM. Optimized CRISPR tools and site-directed transgenesis towards gene drive development in Culex quinquefasciatus mosquitoes. Nat Commun. 2021;12(1):2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E, James AA. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci USA. 2015;112(49):E6736–E6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz VM, Bier E. The dawn of active genetics. Bioessays. 2016;38(1):50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfray H, North A, Burt A. How driving endonuclease genes can be used to combat pests and disease vectors. BMC Biol. 2017;15(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, Gribble M, Baker D, Marois E, Russell S, et al. CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol. 2016;34(1):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond AM, Kyrou K, Bruttini M, North A, Galizi R, Karlsson X, Kranjc N, Carpi FM, D'Aurizio R, Crisanti A, et al. The creation and selection of mutations resistant to a gene drive over multiple generations in the malaria mosquito. PLoS Genet. 2017;13(10):e1007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Calvo E, Marinotti O, Fang J, Rizzi M, James AA, Li J. Analysis of the wild-type and mutant genes encoding the enzyme kynurenine monooxygenase of the yellow fever mosquito, Aedes aegypti. Insect Mol Biol. 2003;12(5):483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou K, Hammond AM, Galizi R, Kranjc N, Burt A, Beaghton AK, Nolan T, Crisanti A. CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat Biotechnol. 2018;36(11):1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs AT, Li F, Jasinskiene N, Chen X, Nirmala X, Marinotti O, Vinetz JM, James AA. Engineered resistance to Plasmodium falciparum development in transgenic Anopheles stephensi. PLoS Pathog. 2011;7(4):e1002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzen B. The Tryptophan → Ommochrome pathway in insects. In: Treherne JE, Berridge MJ, Wigglesworth VB, editors. Advances in Insect Physiology, Vol. 10. London: Academic Press; 1974. p. 117–246. [Google Scholar]
- Lorimer N, Lounibos LP, Petersen JL. Field trials with a translocation homozygote in Aedes aegypti for population replacement. J Econ Entomol. 1976;69(3):405–409. [DOI] [PubMed] [Google Scholar]
- Osanai-Futahashi M, Tatematsu KI, Futahashi R, Narukawa J, Takasu Y, Kayukawa T, Shinoda T, Ishige T, Yajima S, Tamura T, et al. Positional cloning of a Bombyx pink-eyed white egg locus reveals the major role of cardinal in ommochrome synthesis. Heredity (Edinb). 2016;116(2):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TB, Phong CH, Bennett JB, Hwang K, Jasinskiene N, Parker K, Stillinger D, Marshall JM, Carballar-Lejarazú R, James AA. Experimental population modification of the malaria vector mosquito, Anopheles stephensi. PLoS Genet. 2019;15(12):e1008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon JL, Scott TW. Crimson: a novel sex-linked eye color mutant of Culex pipiens L. (Diptera: Culicidae). J Med Entomol. 2004;41(3):385–391. [DOI] [PubMed] [Google Scholar]
- Savvateeva-Popova EV, Popov AV, Heinemann T, Riederer P. Drosophila mutants of the kynurenine pathway as a model for ageing studies. Adv Exp Med Biol. 2003;527:713–722. [DOI] [PubMed] [Google Scholar]
- Simoni A, Hammond AM, Beaghton AK, Galizi R, Taxiarchi C, Kyrou K, Meacci D, Gribble M, Morselli G, Burt A, et al. A male-biased sex-distorter gene drive for the human malaria vector Anopheles gambiae. Nat Biotechnol. 2020;38(9):1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terradas G, Hermann A, James AA, McGinnis W, Bier E. High-resolution in situ analysis of Cas9 germline transcript distributions in gene-drive Anopheles mosquitoes. G3 (Bethesda). 2021;12;jkab369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volohonsky G, Terenzi O, Soichot J, Naujoks DA, Nolan T, Windbichler N, Kapps D, Smidler AL, Vittu A, Costa G, et al. Tools for Anopheles gambiae Transgenesis. G3. 2015;5(6):1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RJ, Cook LM, Hamilton A, Whitelaw A. Transporting the marker gene re (Red Eye) into a laboratory cage population of Aedes aegypti (Diptera: Culicidae), using meiotic drive at the MD locus. J Med Entomol. 1977;14(4):461–464. [DOI] [PubMed] [Google Scholar]
- Xu X, Harvey-Samuel T, Yang J, Alphey L, You M. Ommochrome pathway genes kynurenine 3-hydroxylase and cardinal participate in eye pigmentation in Plutella xylostella. BMC Mol Cell Biol. 2020;2:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lin Y, Shen G, Tan X, Lei C, Long W, Liu H, Zhang Y, Xu Y, Wu J, et al. Pigmentary analysis of eggs of the silkworm Bombyx mori. J Insect Physiol. 2017;101:142–150. [DOI] [PubMed] [Google Scholar]
- Zhuravlev AV, Vetrovoy OV, Ivanova PN, Savvateeva-Popova EV. 3-Hydroxykynurenine in regulation of Drosophila behavior: the novel mechanisms for Cardinal phenotype manifestations. Front Physiol. 2020;11:971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All mosquito strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Supplemental material is available at GENETICS online.
RC-L, TT, TBP, and AAJ designed experiments; RC-L, TT, and TBP conducted experiments; RC-L, TT, TBP, and AAJ analyzed data, and RC-L, TT, TBP, and AAJ prepared the manuscript.