Fig. 2.
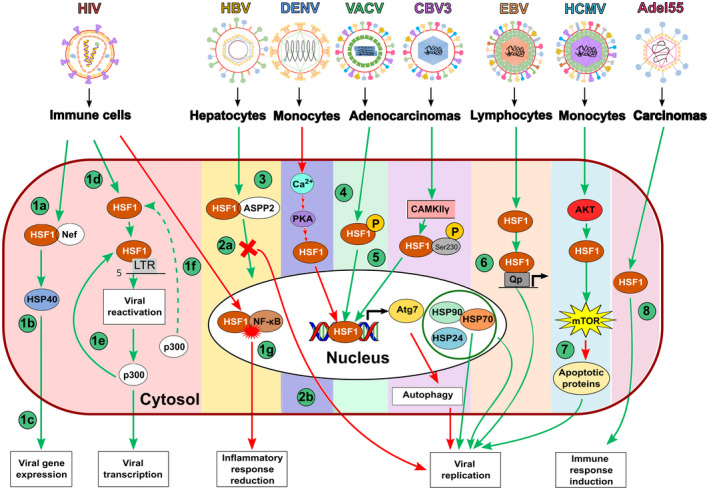
Schematic representations of the participation of HSF1 in viral infections. Red arrows indicate inhibitory pathways, while green arrows indicate activation pathways. From left to right: (1a) HSF1 associates with Nef, an early viral protein produced during HIV‐1 infection and (1b) activates HSP40, which promotes (1c) viral gene expression. (1d) HSF1 promotes the reactivation of HIV from latency, by binding to the 5'LTR in the viral genome and (1e) promotes the recruitment of protein complexes, such as p300. (1f) Additionally, HSF1 recruits p300 for self‐acetylation. (1g) HSF1 acts as a repressor in HIV‐induced inflammation, which occurs through a competition between HSF1 and nuclear factor κB (NF‐κB), which inhibits the NF‐κB pathway. (2a) HSF1 binds to ASPP2, which blocks the translocation of HSF1 to the nucleus and impairs Atg7 transcription, (2b) thus preventing autophagy and the replication of the hepatitis B virus (HBV) in hepatocytes. (3) HSF1 promotes autophagy through the transcription of Atg7 and inhibits dengue virus (DENV) replication. (4) HSF1 and heat shock proteins (HSPs), such as HSP90, HSP70, and other HSPs promote the replication of vaccinia virus (VACV). (5) Coxsackievirus B3 (CVB3) activates HSF1 and promotes the transcription of the gene of HSP70 through which downstream interactions promote viral replication. (6) There is a heat shock response element (HSE) in the viral genome of Epstein–Barr virus (EBV), specifically in the Qp gene. HSF1 binds to Qp promoting the initiation of viral replication in EBV‐infected cells. (7) The human cytomegalovirus (HCMV) promotes HSF1 activation to inhibit apoptosis, thus extending the lifespan of infected monocytes. (8) Finally, a constitutively active mutant of HSF1 (cHSF1) induces viral replication, and its overexpression induces a tumor‐specific immune response when using the oncolytic adenovirus Adel55.
