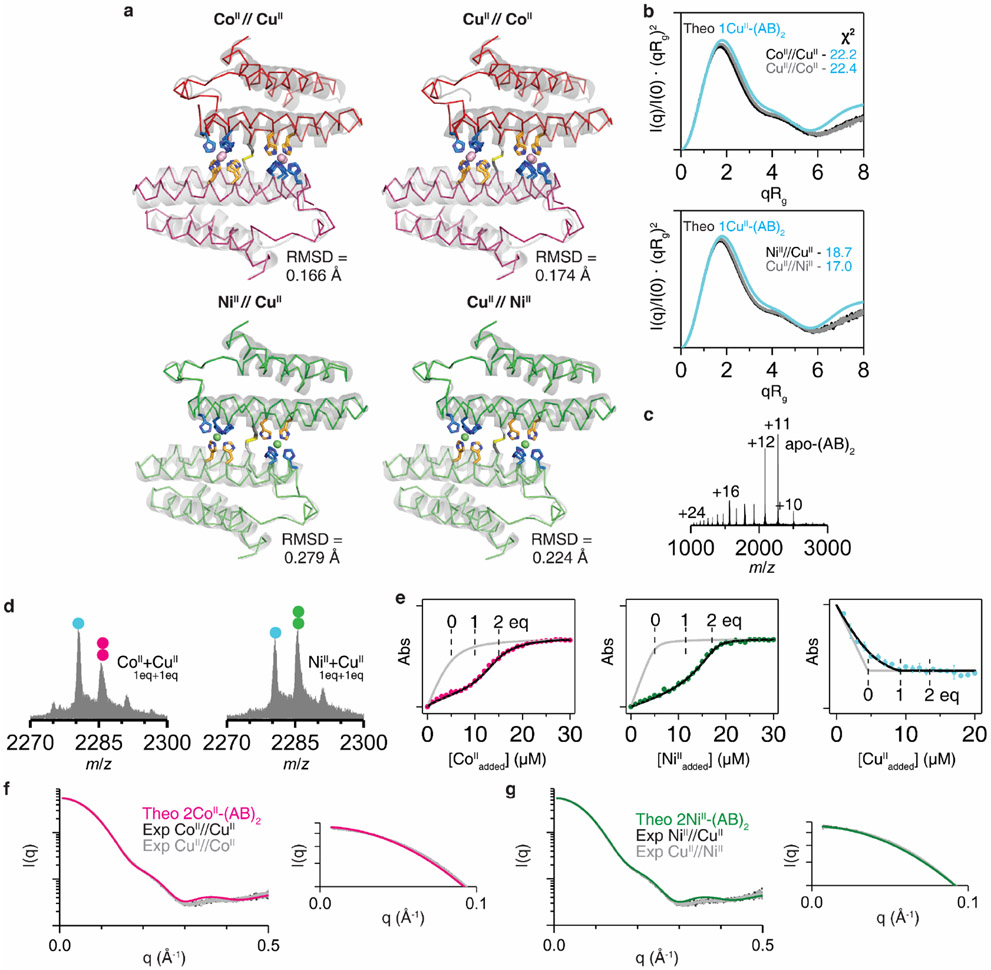
Extended Data Figure 3 ∣. Structural characterization and metal-binding analysis of MII-(AB)2 complexes under competitive conditions with CuII.
a, Crystal structures (ribbon models) of (AB)2 formed under CoII//CuII (PDB:7RRB), CuII//CoII (PDB:7RRR), NiII//CuII (PDB:7LR5), and CuII//NiII (PDB:7LRA) conditions. Root mean square deviation (RMSD) values were determined in comparison with the crystal structures of 2CoII-(AB)2 and 2NiII-(AB)2 (grey cartoon models). b, Experimental SAXS profile of (AB)2 (0.8 mM) in CoII//CuII, CuII//CoII, NiII//CuII, and CuII//NiII conditions ([MII] = 1.6 mM) compared with the theoretical SAXS profile of 1CuII-(AB)2. c, ESI-MS spectrum of (AB)2 (5 μM) without metal ions. d, ESI-MS spectrum of (AB)2 (5 μM) with sub-stoichiometric amounts of CuII (5 μM) and CoII or NiII (5 μM). Circles in ESI-MS spectra represent the number of CoII (magenta), NiII (green), and CuII (cyan) ions bound to (AB)2. e, Competitive metal-binding titration of (AB)2 with CoII (magenta), NiII (green), and CuII (cyan) in 20 mM NH4HCO3 (pH 7.8). Mag-Fura-2 (5 μM) was used for competitive CoII and NiII titration, and Newport green DCF (5 μM) was used for competitive CuII titration. Experimental data points and error bars are presented as mean and standard deviation of three independent measurements. Log-scale plots (left) of Fig. 2c to compare experimental scattering profiles of f, CoII//CuII and CuII//CoII, and g, NiII//CuII and CuII//NiII with theoretical scattering profiles. Right panels present expanded low q-ranges of the scattering plots (left).