Abstract
Background
The objectives of this study were to delineate whether delirium in older adults is associated with activation of the immune-inflammatory response system (IRS) as indicated by activation of M1, T helper (Th)1, and Th17 profiles, and/or by reduced activities of the compensatory immunoregulatory system (CIRS), including Th2 and T regulatory profiles.
Methods
We recruited 65 older adult patients with a low energy impact hip fracture who underwent hip fracture operation. The CAM-ICU and the Delirium Rating Scale, Revised-98-Thai version (DRS-R-98) were assessed pre-operatively and 1, 2 and 3 days after surgery. Blood samples (day 1 and 2) post-surgery were assayed for cytokines/chemokines using a MultiPlex assay and the neutrophil/lymphocyte ratio.
Results
We found that delirium and/or the DRS-R-98 score were associated with IRS activation as indicated by activated M1, Th1, Th17 and T cell growth profiles and by attenuated CIRS functions. The most important IRS biomarkers were CXCL8, interleukin (IL)-6, and tumor necrosis factor-α, and the most important CIRS biomarkers were IL-4 and soluble IL-1 receptor antagonist. We found that 42.5% of the variance in the actual changes in the DRS-R-98 score (averaged from day 1 to day 3) was explained by T cell growth factors, baseline DRS-R-98 scores and age. An increase in the NLR reflects overall IRS, M1, Th1, Th17, and Th2 activation.
Conclusions
Post-hip surgery delirium is associated with activated IRS pathways and appears especially in patients with lowered CIRS functions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-022-04021-y.
Keywords: Delirium, Hip fracture, Inflammation, Cytokines, Biomarkers, Neuroimmune, Neurocognition
Background
Delirium is a neuropsychiatric syndrome presenting with altered levels of attention, awareness, and cognitive functions [1, 2]. Delirium is commonly observed in hospitalized older adults who require surgical management for hip fracture surgery [3–5] with a prevalence of pre-operative [6, 7] and post-operative hip fracture delirium of 12.7–57.6% and 16.9–24%, respectively [8, 9]. Increased risk of medical comorbidities, prolonged length of intensive care unit and hospital stay, as well as higher mortality are documented in hip fracture patients with delirium [10–12].
During the acute period of severe physical illness, delirium may develop as the final pathway of intertwined systemic and central nervous system (CNS) pathogenic processes [13, 14]. The aging brain, circadian rhythm disturbances, stress responses including in the endocrine system, increased oxidative stress, neurotransmitter dysregulations, neuronal circuit disruptions, as well as activation of immune-inflammatory pathways contribute to the pathophysiology of delirium [15, 16]. We found a significant association between the onset of delirium following hip fracture and increased white blood cell number, neutrophil percentage, neutrophil / lymphocyte ratio (NLR), and blood gas parameters including elevated pO2 values [17]. The most significant biomarker of delirium was an increase in the NLR [17], indicating that delirium due to hip fracture may be caused by an aseptic immune-inflammatory process originating from hip tissue trauma which triggers a more generalized immune response. In this respect, previous reports showed that increases in peripheral levels of C-reactive protein (CRP), interleukin-6 (IL-6), CXCL8 (IL-8), and tumor necrotic factor (TNF)-α are associated with the onset of post-operative delirium [3, 18–20]. However, not all research came to the same conclusion all of the time. For example, one study showed increased IL-6, IL-2, and IL-1α levels in delirium due to septic shock, although no such increases in CRP, CXCL8 and TNF-α could be established [21].
Overall, the findings reflect peripheral activation of immune-inflammatory pathways which is triggered by local tissue injury and/or surgical damage ultimately leading to neuro-inflammatory signaling in the brain which may contribute to the symptoms of delirium [22]. It is interesting to note that mild chronic activation of immune-inflammatory pathways occurs in schizophrenia [23, 24], affective disorders [25, 26], and dementia [27], which are known risk factors of delirium [28]. Moreover, increased neurotoxicity due to the cumulative effects of M1 macrophage, T helper (Th)1, and Th17 phenotypes and neurotoxic cytokines/chemokines e.g., CCL11, CCL2, RANTES (CCL5), CXCL10 (IP-10) and CCL3 (macrophage inflammatory protein 1α) to a large extent explain the symptoms and cognitive impairments of the major psychoses [24, 29–32] and, therefore, could be involved in the pathophysiology of delirium.
Moreover, the compensatory immune regulatory system (CIRS), which may attenuate an overzealous inflammatory response, is activated in affective disorders and schizophrenia and these include T regulatory (Treg) (e.g., IL-10) and Th2 (e.g., IL-4, IL-9, and IL-13) profiles [31, 32]. However, there are no data whether delirium is associated with M1 macrophage, Th1, Th2, Th17, Treg or CIRS cytokine profiles, and whether a neurotoxic cytokine profile including M1, Th1, Th17 and neurotoxic chemokines, such as CCL11, CCL2, CCL3, CCL5, and CXCL10) is associated with delirium.
Hence, the aims of this study were to delineate a) the cytokine profiles (including M1 macrophage, Th1, Th2, Th17, Treg, Tcell growth) of delirium due to hip fracture; b) whether a neurotoxic cytokine/chemokine profile consisting of M1, Th1, Th17 cytokines and the neurotoxic CCL11, CCL2, CCL3, CCL5, CXCL8 and CXCL10 chemokines are associated with delirium. The specific hypothesis is that delirium is characterized by increased M1, Th1, and Th17-neurotoxicity profiles and lowered CIRS, Treg and Th2 profiles. Moreover, since the NLR is a major biomarker of delirium we also examined the cytokine profiles, which are associated with the increased NLR.
Methods
Participants
We recruited sixty-five older adults with hip fracture who were admitted into the Hip Fracture Pathway Inpatient Care at King Chulalongkorn Memorial Hospital, Bangkok, Thailand between June, 2019 and February, 2020. Patients aged 65-year and older and who suffered from a low energy impact hip fracture and underwent a hip fracture operation and were postoperatively transferred to the surgery intensive care unit (SICU) or orthopedic units were included into the study. The diagnosis of delirium was made using the Confusion Assessment Method-Intensive Care Unit-Thai version (CAM-ICU) [33] and DSM-5 criteria of delirium [1]. Exclusion criteria were: a high energy impact hip fracture, metastatic fractures, intracranial vascular lesion and other traumatic brain injury from falling, coma or premorbid dementia, a life-time history of (neuro)-inflammatory and/or neurodegenerative disease including multiple sclerosis, Parkinson’s and Alzheimer’s disease, rheumatoid arthritis, inflammatory bowel disease, and major psychiatric illness such as schizophrenia, bipolar disorder, and the acute phase of a major depressive disorder. Remitted patients with major depression, stroke patients one year after the acute stroke and subjects with mild cognitive impairment could be included. We also excluded patients who could not communicate in Thai language.
Clinical assessments
Initially, the demographic and clinical information of the research participants was extracted from the electronic medical records and bedside interviews. The baseline cognitive status and delirium scores were assessed within 24 h before the surgery date. Then, we re-assessed daily the cognitive status and delirium severity and diagnosis during three consecutive days postoperatively. The CAM-ICU and Delirium Rating Scale, Revised-98-Thai version (DRS-R-98) were used at bedside to determine the presentation and the severity of the delirium in the evening of day 0 (pre-operative day), and twice a day the three consecutive days after surgery. Both CAM-ICU-T and DRS-R-98-T show good sensitivity, specificity for delirium and interrater reliability [33, 34]. We recorded the use of anticholinergic medications, benzodiazepines, opiates, and psychotropic drugs prior to hospitalization, as well as pertinent peri/post-operative clinical data, such as operative time, blood loss, and the need for restraint due to psychomotor agitation.
The Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (registration number 528/61) institutional review board reviewed and approved this study in accordance with the International Guideline for the Protection of Human Subjects, as required by the Declaration of Helsinki, The Belmont Report, CIOMS Guideline, and International Conference on Harmonization in Good Clinical Practice (ICH-GCP). All patients and their guardians (first degree family members) provided written informed consent.
Assays
Along with the clinical evaluation, venous blood samples were collected post-surgery, daily at 7.00 am, for two consecutive days. Blood samples were sent to the laboratory to assay complete blood counts and venous blood gas. Plasma and serum samples were frozen at -80 °C until thawed for the assay of cytokines/chemokines. The CBC (NLR) and blood gas (HCO3-) assays were performed as described previously [17]. The CBC values were determined using a flow cytometry method with a semiconductor laser (Sysmex, Kobe, Japan) and the NLR were determined from the CBC results. The NLR was calculated as a z unit-based composite score equal to the difference between the z scores of neutrophil and z scores of lymphocyte percentages. The blood gas data were analyzed using an ion-selective electrode from the Nova Stat Profile pHOx series (Nova Biomedica, MA, USA).
To assay cytokines/chemokines, we used the Bio-Plex Pro™ Human Chemokine Assays (Bio-Rad Laboratories, Inc. USA). We assayed IL-1β, IL-1Ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, basic FGF, CCL11 (eotaxin), G-CSF, GM-CSF, IFN-γ, CXCL10, CCL2, CCL3, CCL4, PDGF, CCL5, TNF-α, and VEGF. Fifty microlitres of serum (1:4 dilutions in sample diluent HB) was mixed with 50 µl of microparticle cocktail (containing cytokine/chemokines per well of a 96-well plate provided by the manufacturer) and incubated for 1 h at room temperature while shaking at 850 rpm. Wells were washed three times before another 50 µl of diluted Streptavidin-PE was added and further incubated for 10 min at room temperature on shaker at 850 rpm. Finally, wells were washed three times and 125 µl of assay buffer was added and shake at 850 rpm at room temperature for 30 s before being read with Bio-Plex® 200 System (Bio-Rad Laboratories, Inc.). In the data analyses we used the concentrations of the cytokines/chemokines. More than 20% of all measured concentrations of IL-2, IL-5, IL-10, IL-12, IL-13, IL-15, GM-CSF and VEGF were below the detection limit and, therefore, these cytokines/growth factors were excluded from the analyses concerning the effects of single cytokines/growth factors. Nevertheless, these values were considered when computing immune profiles because measurable levels of those cytokines/growth factors may contribute to IRS/CIRS/ T cell growth responses. The primary outcome variables in this study were different immune profiles, namely the M1 macrophage profile computed as z IL-1β + z IL-6 + zTNF-α + z CXCL8 + z CCL3 + z sIL-RA; Th1 as z IL-2 + z IL-12 + z IFN (interferon)-γ; Th2: z IL-4 + z IL-9 + z IL-13; Th17: z IL-6 + z IL-17; the IRS/CIRS ratio as z (M1 + Th1 + Th-17) – z (z IL-4 + z IL-9 + z IL-13 + z IL-10); T cell growth (all factors that promote T cell growth): z IL-4 + z IL-7 + z IL-9 + z IL-12 + z IL-15 + z GM-CSF (granulocyte–macrophage colony-stimulating factor) [31, 32]. Neurotoxicity was conceptualized as a composite score comprising neurotoxic cytokines/chemokines: z IL-1β + z TNF-α + z IL-6 + z IL-2 + z IFN-γ + z IL-17 + zCCL11 + z CXCL10 + z CCL3 + z CCL5 + z CCL2 [31, 32]. The intra-assay CV values for all analytes were < 11.0%.
Statistics
The X2-test was used to determine associations between sets of categorical variables, and analysis of variance (ANOVA) was used to determine between-group differences in scale variables. The primary outcome measures are the delirium diagnosis, as determined by the CAM-ICU and DSM-5, and the quantitative DRS-R-98 scale scores. The primary statistical analyses used generalized estimating equations (GEE) to examine the associations between the immune profiles and NLR on the outcome (delirium as binary variable), or multiple regression analyses which examined the effects of immune profiles, HCO3- and clinical variables (previous MDD and CNS disease, age, sex, BMI, time to surgery, estimated blood loss during surgery, duration of surgery, use of deliriogenic medications, insomnia, nasal cannula oxygen) on the DSR-R-98 scores while adjusting for the baseline DRS-R-98 scores. The latter regression analysis estimates the effects of biomarkers on the actual changes in DRS-R-98 score from baseline to day 1, 2 or 3. Multiple comparisons among treatment means or multiple associations between outcome data and immune profiles were adjusted using False Discovery Rate (FDR) p-correction. Automatic multivariate regression analysis was employed to predict dependent variables (the DRS-R-98 scores) using immune profiles, NLR and chemokines and demographic data, while examining R2 changes, multicollinearity (using tolerance and VIF), multivariate normality (Cook's distance and leverage), and homoscedasticity (using White and modified Breusch-Pagan tests for homoscedasticity). We used an automatic stepwise (step-up) procedure with a 0.05 p-to-enter and a 0.06 p-to-remove. The results of all these regression analyses were always bootstrapped using 5.000 bootstrap samples, and the latter are shown if the results were not concordant. IBM SPSS Windows version 25, 2017 was used for statistical analysis and statistical significance was set at < 0.05 (two tailed tests). Using G power analysis, the sample size for a repeated measurement design ANOVA should be around n = 60 when the effect size is 0.3, alpha is 0.05, power is 0.80, including two groups (delirium versus non-delirium) and two repeated (the biomarkers) measurements. The a priori computed required sample size for a multiple regression analysis given an effect size of 0.3, alpha 0.05, power 0.80, number of predictors five is around forty-nine. Therefore, in the present study sixty-five patients were included.
Results
A total of one hundred older adult patients with hip fracture who underwent orthopedic surgery were hospitalized during the study enrollment period. Twelve patients refused to participate in the study. Twenty-three patients were excluded from the study due to history of dementia, severe hearing impairment, unable to communicate in Thai, pathologic fracture, recent stroke, and an active major depressive episode. Eventually, sixty-five participants participated in the study and nineteen of them (29.2%) developed delirium peri-operatively. The study flow chart is shown in the Supplementary File 1. Five subjects showed delirium pre-surgery, six showed new-onset delirium on day 1, two on day 2 and three on day 3, while three subjects showed delirium during the whole study.
Table 1 shows the socio-demographic and clinical data in both patients with and without delirium. The delirious patients showed a higher mean age and lower body mass index (BMI), and a longer waiting time to surgery. There were no differences in sex ratio, marital status, surgical time, blood loss during surgery and the pain scores between both study groups. There were no significant between-group differences in HCO3-, insomnia, and pre-hospitalization use of deliriogenic drugs including tricyclic antidepressants, benzodiazepines, Z-drugs, opioid medications, anticholinergics, and first-generation antihistamines, while the prevalence of CNS disease (such as traumatic brain injury, benign brain tumor, mild cognitive impairment) and previous stroke was higher in those with a delirium.
Table 1.
Socio-demographic and clinical data of hip surgery patients divided into those with and without delirium
| Variables | No Delirium (N = 46) | Delirium (N = 19) | F/X2 | df | p |
|---|---|---|---|---|---|
| Age (years) | 79.7 (7.9) | 84.5 (6.3) | 5.57 | 1/63 | 0.021 |
| Sex (female/male) | 36/10 | 15/4 | 0.00 | 1 | 0.951 |
| Education (years) | 7.5 (5.6) | 9.4 (6.9) | 1.44 | 1/61 | 0.235 |
| BMI (kg/m2) | 22.3 (3.2) | 19.7 (2.7) | 8.42 | 1/57 | 0.005 |
| Marital status (single/married/divorced) | 5/20/1 | 6/6/0 | FEPT | - | 0.233 |
| Time to surgery (hours) | 60.8 (47.9) | 109.1 (101.5) | 6.79 | 1/62 | 0.011 |
| Surgical time (minutes) | 104.5 (42.8) | 97.9 (29.9) | 0.38 | 1/63 | 0.543 |
| Blood loss (mL) | 199.8 (110.8) | 231.6 (138.7) | 0.95 | 1/63 | 0.333 |
| HCO3− (mEq/L) | 27.1 (3.4) | 26.8 (3.5) | 0.09 | 1/63 | 0.762 |
| Previous CNS disease (N/Y) | 36/10 | 7/12 | 10.30 | 1 | 0.002 |
| Insomnia (N/Y) | 41/5 | 17/2 | 0.00 | 1 | 0.968 |
| Previous stroke (N/Y) | 42/4 | 12/7 | 7.58 | 1 | 0.006 |
| Previous depression (N/Y) | 42/4 | 18/1 | FEPT | - | 1.000 |
| Deliriogenic drugs (N/Y) | 28/18 | 12/7 | 0.03 | 1 | 0.863 |
Results are shown as mean ± SD. All results of analysis of variance (F values), analysis of contingency analysis (X.2) or Fisher exact probability test (FEPT, BMI body mass index
Table 2 shows the results of GEE analysis performed with delirium as dependent variables and the immune profiles and NLR as explanatory variables. The changes in the DRS-R-98 scores from baseline to the mean values at day 1, 2 and 3 were significantly higher in delirium patients as compared with those without delirium. After FDR p-correction, the IRS/CIRS ratio, M1, Th17, T cell growth, and NLR were significantly and positively associated with delirium (no delirium as reference group).
Table 2.
Differences in Delirium Rating Scale, Revised-98-Thai (DRS) and pain scores and immune profiles between patients with and without delirium
| Clinical scales (days 1–3) | No Delirium | Delirium | B | SE | Wald X2 | df | p |
| Mean DRS days 1–3* | -0.176 (0.086) | 0.427 (0.229) | 0.604 | 0.2466 | 6.00 | 1 | 0.014 |
| Immune profiles (days 1–2) all in z scores | No Delirium | Delirium | B | SE | Wald X2 | df | pFDR |
| IRS/CIRS | -0.172 (0.132) | 0.450 (0.132) | 0.622 | 0.186 | 11.16 | 1 | 0.0035 |
| M1 macrophage | -0.145 (0.128) | 0.377 (0.160) | 0.532 | 0.205 | 6.50 | 1 | 0.026 |
| Th1 | -0.071 (0.141) | 0.176 (0.169) | 0.246 | 0.220 | 1.26 | 1 | 0.305 |
| Th2 | -0.125 (0.122) | 0.275 (0.241) | 0.400 | 0.271 | 2.19 | 1 | 0.194 |
| Th17 | -0.191 (0.119) | 0.473 (0.165) | 0.664 | 0.203 | 10.67 | 1 | 0.0035 |
| T cell growth | -0.122 (0.168) | 0.481 (0.201) | 0.626 | 0.262 | 5.70 | 1 | 0.029 |
| Neurotoxicity | -0.053 (0.150) | 0.143 (0.222) | 0.196 | 0.268 | 0.54 | 1 | 0.463 |
| z NLR | -0.319 (0.249) | 0.773 (0.265) | 1.903 | 0.364 | 8.99 | 1 | 0.003 |
M1 macrophage, Th1 T helper, Treg T regulatory profile T cell growth a z composite score comprising T cell growth factors, neurotoxicity a z composite score based on neurotoxic cytokines/chemokines, IRS/CIRS ratio of immune response system / compensatory immunoregulatory system, z NLR neutrophil / lymphocyte ratio (computed as a z composite score)
*Results of GEE analysis with the mean DRS-R-98 score (averaged over days 1, 2 and 3) as dependent variable and basal DRS-R-98 score, age, sex, body mass index, previous depression and central nervous system disease as explanatory variables
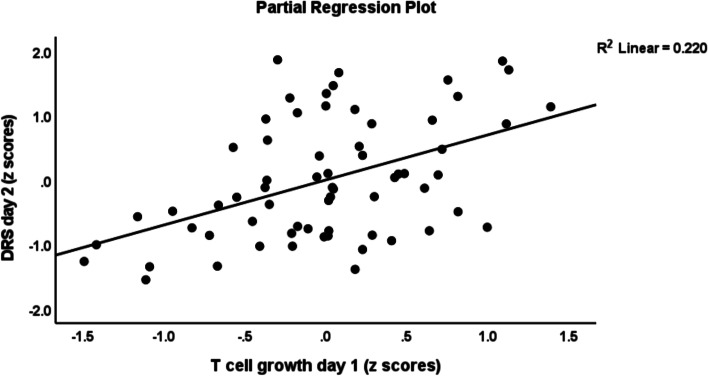
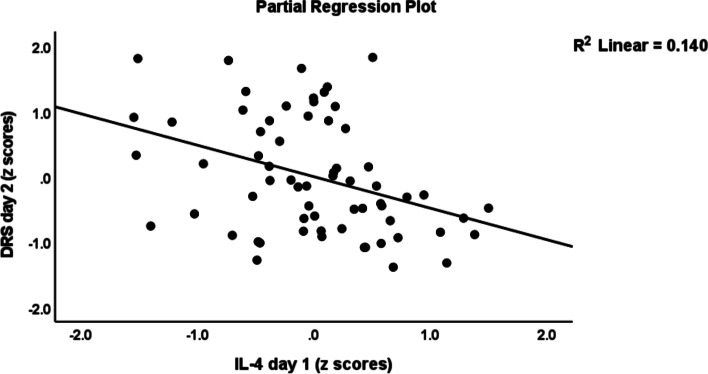
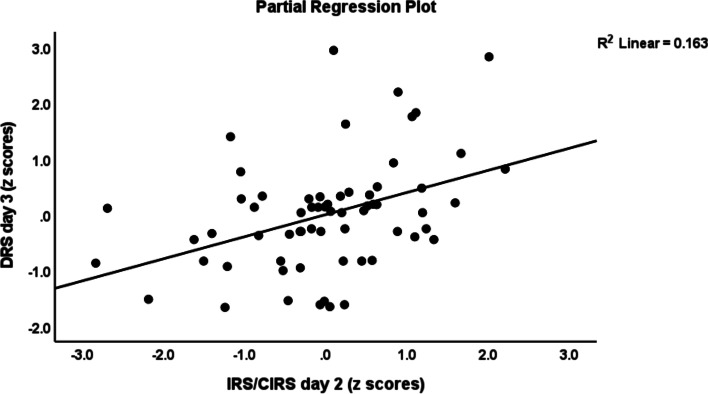
Table 3 shows the results of multiple regression analyses with the DRS-R-98 scores at day 1, day 2 and day 3 as dependent variables and cytokine profiles as explanatory variables while allowing for the effects of demographic/clinical data. Regression # 1 shows that 33.0% of the variance in the DRS-R-98 score at day 1 was explained by CNS disease and IL-8 (day 1) (both positively associated) and BMI (inversely associated). Regression # 2 shows that 31.3% of the variance in the DRS-R-98 score at day 2 was explained by the cumulative effects of T cell growth profile (positively associated) and BMI and IL-4 (both inversely). Figure 1 shows the partial regression of the DRS-R-98 score (day 2) on the T cell growth profile measured at day 1 after controlling for the variables shown in regression # 2. Figure 2 shows the partial regression of the DRS-R-98 score (day 2) on IL-4 (day 1). Regression # 3 shows that 35.5% of the variance in the DRS-R-98 score (day 2) was explained by a combination of Th1 profile (day 2), age, CNS disorders (positively), and sIL-1RA (day 2 an inversely). Regression # 4 shows that 15.9% of the variance in the DRS-R-98 score (day 3) was positively associated with the IRS/CIRS ratio at day 2. Figure 3 shows the partial regression of the DRS-R-98 score (day 3) on the IRS/CIRS ratio at day 2 after controlling for age (not significant). Regression #5 shows that 15.3% of the variance in the mean DRS-R-98 score averaged over days 1, 2 and 3 was explained by the regression on IL-6 (day 1 and positively) and BMI (inversely).
Table 3.
Results of multiple regression analysis with the Delirium Rating Scale, Revised-98-Thai (DRS) scores at day 1, day 2 and day 3 as dependent variables and immune/cytokine profiles as explanatory variables while allowing for the effects of demographic/clinical data
| Dependent variables | Explanatory variables | β | t | p | F model | df | p | R2 |
|---|---|---|---|---|---|---|---|---|
| # 1 DRS day 1 | Model | 10.03 | 3/61 | < 0.001 | 0.330 | |||
| CNS disease | 0.450 | 4.26 | < 0.001 | |||||
| IL-8—day 1 | 0.263 | 2.51 | 0.015 | |||||
| BMI | -0.262 | -2.49 | 0.016 | |||||
| #2 DRS day 2 | Model | 9.13 | 3/60 | < 0.001 | 0.313 | |||
| BMI | -0.283 | -2.61 | 0.011 | |||||
| T cell growth—day1 | 0.732 | 4.13 | < 0.001 | |||||
| IL-4 – day 1 | -0.558 | -3.13 | 0.003 | |||||
| #3 DRS day 2 | Model | 7.84 | 4/57 | < 0.001 | 0.355 | |||
| Th1—day 2 | 0.397 | 3.54 | < 0.001 | |||||
| Age | 0.370 | 3.19 | 0.002 | |||||
| sIL-1RA—day 2 | -0.289 | -2.48 | 0.016 | |||||
| CNS disease | 0.228 | 2.12 | 0.039 | |||||
| #4 DRS day 3 | Model | 11.88 | 1/63 | < 0.001 | 0.159 | |||
| IRS/CIRS—day 2 | 0.398 | 3.45 | < 0.001 | |||||
| #5 Mean DRS days 1—3 | Model | 5.51 | 2/61 | 0.006 | 0.153 | |||
| IL-6—day 1 | 0.313 | 2.65 | 0.010 | |||||
| BMI | -0.244 | -2.07 | 0.043 |
CNS Central nervous system, IL interleukin, BMI body mass index, Th T helper, IRS/CIRS immune-inflammatory response system / compensatory immunoregulatory system ratio
Fig. 1.
Partial regression of the Delirium Rating Scale, Revised-98-Thai (DRS) score (day 2) on the T cell growth profile measured at day 1
Fig. 2.
Partial regression of the Delirium Rating Scale, Revised-98-Thai (DRS) score (day 2) on interleukin (IL)-4 levels measured day 1
Fig. 3.
Partial regression of the Delirium Rating Scale, Revised-98-Thai (DRS) score (day 3) on the immune-inflammatory responses system (IRS) / compensatory immunoregulatory system (CIRS) ratio measured day 2
Consequently, we have delineated the immune profiles and cytokines/chemokines that predict the DRS-R-98 scores at day 1, day 2 or day 3 after adjusting for the baseline DRS-R-98 values. As such these multiple regression analyses examine the associations between the residualized DRS-R-98 scores (i.e. the actual changes from baseline to days 1. 2 or 3) and the immune profiles with and without demographic/clinical data. Table 4 regression # 1 shows that 59.9% of the variance in DRS-R-98 score (day 1) was explained by the regression on DRS baseline, IL-8, TNF-α and T cell growth (all day 1 and positively associated) and CIRS and IL-4 (all day 1 and inversely associated). Regression # 2 shows that 38.4% of the variance in the DRS-R-98 score (day 2) was explained by the regression on the DRS baseline and Th1 (day 2) (both positively) and IL-4 (day 2, inversely associated). Regression # 3 shows that 29.7% of the variance in DRS-R-98 score at day 2 was explained by the regression on age, Th1 (day 1) and DRS baseline (all positively). Regression # 4 shows that 27.9% of the variance in the DRS-R-98 score at day 3 was predicted by the IRS/CIRS ratio (day 2) and baseline DRS (both positively associated). Regression #5 shows that 42.5% of the variance in the mean DRS-R-98 score averaged over days 1, 2 and 3 was explained by DRS baseline, age and T cell growth (day 1) (all positively). The neurotoxicity profile, NLR and HCO3- were not significant in any of the above regressions.
Table 4.
Result of multiple regression analysis with the Delirium Rating Scale, Revised-98-Thai (DRS) scores at day 1, day 2 or day 3 as dependent variables and immune/cytokine profiles and baseline DRS scores as explanatory variables
| Dependent variables | Explanatory variables | B | t | p | F model | df | p | R2 |
|---|---|---|---|---|---|---|---|---|
| #1 DRS day 1 | Model | - | - | - | 14.42 | 6/58 | < 0.001 | 0.599 |
| DRS—baseline | 0.527 | 6.12 | < 0.001 | |||||
| IL-8—day 1 | 0.383 | 3.80 | < 0.001 | |||||
| CIRS—day 1 | -0.362 | -2.42 | 0.019 | |||||
| T cell growth—day 1 | 0.477 | 3.11 | 0.003 | |||||
| IL-4—day 1 | -0.526 | -3.06 | 0.003 | |||||
| TNF-α—day 1 | 0.331 | 2.58 | 0.012 | |||||
| # 2 DRS day 2 | Model | 12.04 | 3/58 | < 0.001 | 0.384 | |||
| DRS—baseline | 0.413 | 3.98 | < 0.001 | |||||
| Th1—day 2 | 0.486 | 4.33 | < 0.001 | |||||
| IL-4—day 2 | -0.236 | -2.09 | 0.041 | |||||
| #3 DRS day 2 | Model | 8.46 | 3/60 | < 0.001 | 0.297 | |||
| Age | 0.2665 | 2.21 | 0.031 | |||||
| Th1—day 1 | 0.288 | 2.65 | 0.010 | |||||
| DRS—baseline | 0.275 | 2.29 | 0.026 | |||||
| #4 DRS day 3 | Model | 11.99 | 2/62 | < 0.001 | 0.279 | |||
| IRS/CIRS—day 2 | 0.369 | 3.41 | 0.001 | |||||
| DRS—baseline | 0.348 | 3.22 | 0.002 | |||||
| #5 Mean DRS days 1–3 | Model | 14.78 | 3/60 | < 0.001 | 0.425 | |||
| DRS—baseline | 0.414 | 3.83 | < 0.001 | |||||
| Age | 0.300 | 2.77 | 0.008 | |||||
| T cell growth—day 1 | 0.222 | 2.26 | 0.027 |
IL interleukin, IRS/CIRS immune-inflammatory response system / compensatory immunoregulatory system, TNF tumor necrosis factor, Th T helper
Table 5 shows the results of GEE analyses with the NLR as dependent variable and immune profiles as explanatory variables. We found that the NLR was significantly and positively predicted by the IRS/CIRS ratio, and M1, Th1, Th17, Th2, T cell growth and neurotoxicity profiles.
Table 5.
Association between the neutrophil/lymphocyte ratio and immune profiles
| Immune profiles | B | SE | Wald/X2 | df | p | FDRp |
|---|---|---|---|---|---|---|
| IRS/CIRS | 0.346 | 0.0789 | 19.22 | 1 | < 0.001 | 0.0017 |
| M1 macrophage | 0.433 | 0.0682 | 40.23 | 1 | < 0.001 | 0.0017 |
| Th1 | 0.232 | 0.0823 | 7.98 | 1 | 0.005 | 0.0058 |
| Th17 | 0.421 | 0.0719 | 34.36 | 1 | < 0.001 | 0.0017 |
| Th2 | 0.226 | 0.0741 | 9.28 | 1 | 0.002 | 0.0028 |
| Tcell growth | 0.295 | 0.0848 | 12.08 | 1 | < 0.001 | 0.0017 |
| Neurotoxicity | 0.179 | 0.0698 | 6.58 | 1 | 0.010 | 0.010 |
All results of generalized estimating equations with the neutrophil / lymphocyte ratio as dependent variable
M1 macrophage, Th T helper, IRS/CIRS immune-inflammatory response system / compensatory immunoregulatory system
Discussion
The first major finding of this study is that delirium and/or the severity of delirium symptoms are significantly associated with the IRS/CIRS ratio, namely positively with M1 (i.e. IL-6, CXCL8 and TNF-α), Th1 and Th17 activation and T cell growth (positively), and inversely with IL-4 and sIL-1RA. These findings extend those of previous papers showing that alterations in peripheral levels of IRS cytokines, namely IL-1β, IL-6, CXCL8, IL-10, and TNF-α, and also C-reactive protein (CRP) and NLR are associated with the onset of delirium [3, 18, 19, 35, 36].
M1-associated cytokines including IL-1β, IL-6, CXCL8, and TNF-α, play a key role in the immune response to injuries. Danger associated molecular patterns, endogenous molecules released from death cells, induce local monocytes and macrophages to secrete IL-1β [37], which is a major pro-inflammatory cytokine secreted from sterile injurious areas [38]. Increased IL-1β signaling is involved in many medical and psychiatric conditions such as tissue damage [39], sepsis [40], rheumatoid arthritis [41], as well as schizophrenia [24], and mood disorders [32]. IL-6 produced by locally damaged tissue and macrophages pleiotropically regulates CD4 + T cell differentiation including Th17 proliferation and Treg inhibition [42]. At the local injurious site, macrophages also secrete CXCL8 to promote further local and systemic inflammatory processes including neutrophil stimulation [43]. Accumulation and migration of mononuclear and polymorphonuclear cells (such as neutrophils, macrophages, lymphocytes, monocytes) to the fracture site are observed after a traumatic injury event [44]. Consequently, these immune cells and the injured tissues secrete several pro-inflammatory cytokines and chemokines which expand the inflammatory/anti-inflammatory processes from the local to the systemic level and, subsequently, to the brain [45]. In this respect, TNF-α is one of these potent pro-inflammatory markers which orchestrate acute inflammatory cascades throughout the body and the central nervous system as well [46, 47]. It is important to note that the above inflammatory markers are all consistently associated with delirium [39, 48–52]. Unsurprisingly, our study also observed significantly increased levels the Th1 (combination of IL-2, IFN-γ and IL-12) and T cell growth and activation factors (a combination of IL-4, IL-7, IL-9, IL-12, IL-15, GM-CSF) in the delirious hip fracture patients compared to the non-delirious group. As such, activated cell-mediated immune pathways are associated with the severity of delirium symptoms in older adult with post-surgery hip fracture.
Based on the above results we may conclude that delirium is accompanied by a cascade of early inflammatory mechanisms which extend from local tissue injury to inflammatory cell activation to cytokine release with increased CRP production and an increased NLR, and neuroinflammation [17]. In response to the acute phase of trauma and inflammation, locally and systematically increased IL-6 secreted from macrophages and T-cells signal the hepatocytes to produce positive acute phase proteins including CRP [53, 54]. Here we report that the NLR is a highly significant biomarker of delirium which strongly reflects IRS, M1, Th2, Th17, and IRS/CIRS activation and Tcell growth as well. Nevertheless, NLR was not a significant explanatory variable after considering the effects of immune profiles or combined effects of cytokines/chemokines and, therefore, immune profiles predict delirium symptoms better than NLR. As such, the present study extends previous research that NLR in peripheral blood and high serum and CSF CRP are consistently reported as biomarkers or predictive risk factors of post-operative delirium [17, 19, 55–58].
IL-17 is an inflammatory mediator that is produced by CD4 + Th17 cells stimulated by IL-6 and IL-1β and by CD8 + T cells and neutrophils [32]. IL-17 plays a key role in chronic inflammatory disorders and autoimmunity, and this cytokine stimulates chemokines (e.g., CXCL1, CXCL2 and CXCL8) and granulopoiesis [59]. Due to its neurotoxic effects, increased IL-17 plays a role in first episode psychosis and schizophrenia [60, 61], and mood [50], and neurodegenerative disorders [51].
Importantly, CXCL8 was significantly associated with the severity of delirium as assessed with the DRS-R-98 scale. Increased levels of peripheral IL-8 are reported in many neuropsychiatric disorders such as major depression, schizophrenia, bipolar disorder, autistic spectrum disorder, and Alzheimer’s disease [52, 62, 63]. In response to intracerebral pro-inflammatory stimuli, IL-8 is released from microglia and consequently attracts neutrophils and leucocytes to expand neuro-inflammation, which may lead to a poorer CNS outcome after a traumatic brain injury or bacterial meningitis [64]. Although increased IL-15 levels in peripheral blood and the CNS play a crucial role in neuro-inflammatory disorders including multiple sclerosis, encephalomyelitis, and intracerebral hemorrhage [65–68], this study could not find any increases in this cytokine in delirium. A study in 2009 found that high IFN-γ was significantly associated with more severe delirium [69] and IFN-γ participates in the macrophage stimulating process during acute inflammation [68, 70].
The second major finding of this study is that the CIRS index and IL-4 and sIL-RA, which display anti-inflammatory effects, are inversely associated with delirium and/or the severity of delirium symptoms. The sIL-RA is secreted by activated macrophages and inhibits pro-inflammatory IL-1β signaling [71] and, therefore, reduced levels of sIL-RA as seen in the current and previous studies [69, 72], suggest lowered negative feedback on pro-inflammatory IL-1 signaling [73]. IL-4, which is secreted by Th2 lymphocytes, basophils, mast cells, and eosinophils, leads to the modification and proliferation of lymphocytes, macrophages, fibroblasts and endothelial cells and promotes anti-inflammatory and immunoregulatory processes, as well as healing of tissues [74]. Interestingly, IL-4 function is associated with neuro-restorative effects after cerebral ischemic and traumatic brain injury events, while reduced IL-4 signaling is associated with cognitive impairments in schizophrenia [75]. Moreover, relatively reduced CIRS activity in first episode psychosis predicts a worse outcome following treatment with antipsychotics [76]. Previous research showed that the anti-inflammatory cytokine IL-10, which is produced by different cells but especially by Treg cells, may regulate the initial pro-inflammatory response in delirium [77, 78]. Overall, lowered anti-inflammatory defenses through lowered CIRS functions appear to contribute to delirium.
The third major finding of this study is that the neurotoxic immune profile comprising neurotoxic cytokines and chemokines was not associated with delirium. This contrasts with the increased neurotoxic profiles established in schizophrenia and associated cognitive impairments, mood disorders, and suicide attempts and ideation [79–81]. At the phenomenological level, post-hip fracture operative delirium has an acute onset and is frequently presenting with more positive psychotic features [82] contrasting with the chronic nature of schizophrenia and mood disorders. Thus, the acute increase in IRS (M1, Th1 and Th17) cytokines in patients with attenuated CIRS functions appears to be the most important factor in delirium.
It is important to note that the increased cytokine levels produced at the traumatic site or peripheral vascular and lymphatic system may pass through a damaged blood brain barrier or circumventricular organs to signal inflammatory cascades in the central nervous system [83, 84]. For example, significantly elevated levels of peripheral cytokines including IL-1β, IL-6, IL-8, TNF-α, and IFN-ɣ are involved in the peripheral to central inflammatory signal transduction [47, 85]. Peripheral inflammatory processes which translate peripheral inflammatory signals into central neuro-inflammation and microglial activation are described in delirium [18] and unipolar depression [86] and psychosis [61].
The results of the present study should be discussed with regard to its limitations. A first limitation is that we did not distinguish between delirium subtypes including the hyperactive, hypoactive, and mixed phenotypes. The different delirium subtypes may have a different pathogenesis and may represent different immune-inflammatory and neurochemical pathways [87]. Second, the biomarkers analyzed in this study reflect the peripheral part of the IRS response and not the neuro-inflammatory changes in the CNS which should be examined by cerebrospinal fluid analyses or brain imaging techniques [88]. Third, although CAM assessments were performed twice a day, it would have been better to perform the CAM every 8 h and, therefore, some delirium cases may have been missed, although not affecting the regression analyses which were the primary. Fourth, some readers may think that the 5-year difference in age between patients with and without delirium could be a limitation of this study and that statistically controlling for age is not a panacea. However, there is some debate as to whether unmatched versus matched samples or matching versus confounding is the best approach in case–control studies [89–91]. Nevertheless, we performed not a case–control study but a prospective cohort study with patients undergoimg the same exposure with the aim to delineate the risk factors of delirium severity including immune profiles, age and comorbidities. Accordingly, the primary analyses of this study are the multiple regression analyses which define the risk factors of delirium severity, while the differences between patients with and without delirium are only shown to present mean values of the measured immune profiles. It is only normal that in our cohort study those with delirium show a higher age, because increasing age is a risk factor (see introduction). Furthermore, matching both groups for age is not the most accurate way to control for confounders but is a method to more precisely estimate parameters with a smaller variance [91]. However, in our prospective cohort design matching for age would induce selection bias through partial restriction by selecting the controls and therefore would cause gains or losses in the multiple regression analyses.. Because the immune response to acute immune stimuli is different among younger and older subjects [92], any selection or matching for age could induce considerable bias in the regression results.
Conclusions
Delirium, the severity of delirium and the changes in the DRS-R-98 score from baseline to post-surgery are associated with IRS activation as indicated by M1, Th1, Th17 and T cell growth profiles. The latter changes predict delirium, especially when CIRS functions including IL-4 and sIL-1RA levels are attenuated. The development of delirium is not associated with a neurotoxic cytokine/chemokine profile as observed in mood disorders and schizophrenia, suggesting that the acute effects of IRS cytokines are sufficient to induce neurocognitive impairments, and psychomotor and psychotic symptoms. Increased NLR expression is indicative of overall immunological and M1/Th1/Th17/Th2/Treg cell activation. The observed dysregulation of IRS and CIRS activity in delirium may be regarded as a new therapeutic target for treating and preventing delirium in older adults. Drugs that inhibit IRS upregulation as well as immunoregulatory agents that promote CIRS function should be researched and trialed in delirium patients.
Supplementary Information
Additional file 1: Figure S1. Study flowchart.
Acknowledgements
We gratefully acknowledge the help of all psychiatry/orthopedic/anesthesiology nursing staff and residents involved in the execution of this study.
Authors’ contributions
PT designed the conception, planned and collected the data, provided a delirium care, interpreted the results, drafted and revised the manuscript. YT planned and collected the data, provided a delirium care, revised the manuscript. ST planned and collected the data, provided an orthopedic care, revised the manuscript. SS prepared, analyzed and interpreted the laboratory specimens, revised the manuscript. MM designed the conception, analyzed and interpreted the data, drafted and revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by a Ratchadapisek Sompoch Endowment Fund of the Faculty of Medicine, Chulalongkorn University (RA62/014).
Availability of data and materials
The dataset generated during and/or analyzed during the current study will be available from Prof. Dr. Michael Maes upon reasonable request and once the dataset has been fully exploited by the authors.
Declarations
Ethics approval and consent to participate
Approval for the study was obtained from the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (registration number 528/61), in compliance with the International Guideline for Human Research protection, as required by the Declaration of Helsinki, was conducted according to Thai and international ethics and privacy laws.
Written informed consent was obtained before the study from the patients or their gardians (first-degree family members).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Paul Thisayakorn, Email: paul.thi@chula.ac.th.
Michael Maes, Email: dr.michaelmaes@hotmail.com.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM–5). 2013. [DOI] [PubMed]
- 2.Lipowski ZJ. Delirium (Acute Confusional States) JAMA. 1987;258(13):1789–1792. doi: 10.1001/jama.1987.03400130103041. [DOI] [PubMed] [Google Scholar]
- 3.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet (London, England) 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosk CA, Mus M, Vroemen JP, van der Ploeg T, Vos DI, Elmans LH, et al. Dementia and delirium, the outcomes in elderly hip fracture patients. Clin Interv Aging. 2017;12:421–430. doi: 10.2147/CIA.S115945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Zhao X, Gao L, Wang Y, Wang J. Incidence and associated factors of delirium after orthopedic surgery in elderly patients: a systematic review and meta-analysis. Aging Clin Experimental Res. 2020;33(6):1493-1506. 10.1007/s40520-020-01674-1. [DOI] [PubMed]
- 6.Agrawal S, Turk R, Burton BN, Ingrande J, Gabriel RA. The association of preoperative delirium with postoperative outcomes following hip surgery in the elderly. J Clin Anesth. 2020;60:28–33. doi: 10.1016/j.jclinane.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Freter S, Dunbar M, Koller K, MacKnight C, Rockwood K. Prevalence and Characteristics of Pre-Operative Delirium in Hip Fracture Patients. Gerontology. 2016;62(4):396–400. doi: 10.1159/000442385. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Zhao X, Dong T, Yang Z, Zhang Q, Zhang Y. Risk factors for postoperative delirium following hip fracture repair in elderly patients: a systematic review and meta-analysis. Aging Clin Exp Res. 2017;29(2):115–126. doi: 10.1007/s40520-016-0541-6. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Yin Y, Jin M, Li B. The risk factors for postoperative delirium in adult patients after hip fracture surgery: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2021;36(1):3–14. doi: 10.1002/gps.5408. [DOI] [PubMed] [Google Scholar]
- 10.Arshi A, Lai WC, Chen JB, Bukata SV, Stavrakis AI, Zeegen EN. Predictors and Sequelae of Postoperative Delirium in Geriatric Hip Fracture Patients. Geriatric Orthopaedic Surg Rehabilitation. 2018;9:2151459318814823. doi: 10.1177/2151459318814823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–1579. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HB, Oldham MA, Sieber FE, Oh ES. Impact of Delirium After Hip Fracture Surgery on One-Year Mortality in Patients With or Without Dementia: A Case of Effect Modification. Am J Geriatric Psychiatry. 2017;25(3):308–315. doi: 10.1016/j.jagp.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oldham MA, Flaherty JH, Maldonado JR. Refining Delirium: A Transtheoretical Model of Delirium Disorder with Preliminary Neurophysiologic Subtypes. Am J Geriatric Psychiatry. 2018;26(9):913–924. doi: 10.1016/j.jagp.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatric Psychiatry. 2013;21(12):1190–1222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis and treatment. Critical Care Clin. 2008;24(4):657–722. doi: 10.1016/j.ccc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Hshieh TT, Inouye SK, Oh ES. Delirium in the Elderly. Clin Geriatr Med. 2020;36(2):183–199. doi: 10.1016/j.cger.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Thisayakorn P, Tangwongchai S, Tantavisut S, Thipakorn Y, Sukhanonsawat S, Wongwarawipat T, et al. Immune, Blood Cell, and Blood Gas Biomarkers of Delirium in Elderly Individuals with Hip Fracture Surgery. Dement Geriatr Cogn Disord. 2021;50(2):161–169. doi: 10.1159/000517510. [DOI] [PubMed] [Google Scholar]
- 18.Maldonado JR. Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. 2018;33(11):1428–1457. doi: 10.1002/gps.4823. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Yu Y, Zhu S. Inflammatory markers in postoperative delirium (POD) and cognitive dysfunction (POCD): A meta-analysis of observational studies. PLoS ONE. 2018;13(4):e0195659. doi: 10.1371/journal.pone.0195659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beloosesky Y, Hendel D, Weiss A, Hershkovitz A, Grinblat J, Pirotsky A, et al. Cytokines and C-reactive protein production in hip-fracture-operated elderly patients. J Gerontol A Biol Sci Med Sci. 2007;62(4):420–426. doi: 10.1093/gerona/62.4.420. [DOI] [PubMed] [Google Scholar]
- 21.Dugan K, Patel BK, Pohlman A, Hall JB, Kress JP, Wolfe KS. Prevalence of Delirium Correlates with Pro-Inflammatory Cytokines in Patients with Septic Shock. D50 critical care: the metamorphosis - pain, sedation, delirium, ICU-acquired-weakness, and palliative care. American Thoracic Society International Conference Abstracts: American Thoracic Society; 2019. p. A6671-A.
- 22.Cunningham C. Systemic inflammation and delirium: important co-factors in the progression of dementia. Biochem Soc Trans. 2011;39(4):945–953. doi: 10.1042/BST0390945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2(3):258–270. doi: 10.1016/S2215-0366(14)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF, et al. The Role of Aberrations in the Immune-Inflammatory Response System (IRS) and the Compensatory Immune-Regulatory Reflex System (CIRS) in Different Phenotypes of Schizophrenia: the IRS-CIRS Theory of Schizophrenia. Mol Neurobiol. 2020;57(2):778–797. doi: 10.1007/s12035-019-01737-z. [DOI] [PubMed] [Google Scholar]
- 25.Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135(5):373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- 26.Brunoni AR, Supasitthumrong T, Teixeira AL, Vieira EL, Gattaz WF, Benseñor IM, et al. Differences in the immune-inflammatory profiles of unipolar and bipolar depression. J Affect Disord. 2020;262:8–15. doi: 10.1016/j.jad.2019.10.037. [DOI] [PubMed] [Google Scholar]
- 27.Holmes C. Review: systemic inflammation and Alzheimer's disease. Neuropathol Appl Neurobiol. 2013;39(1):51–68. doi: 10.1111/j.1365-2990.2012.01307.x. [DOI] [PubMed] [Google Scholar]
- 28.Simone MJ, Tan ZS. The role of inflammation in the pathogenesis of delirium and dementia in older adults: a review. CNS Neurosci Ther. 2011;17(5):506–513. doi: 10.1111/j.1755-5949.2010.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sirivichayakul S, Kanchanatawan B, Thika S, Carvalho AF, Maes M. A New Schizophrenia Model: Immune Activation is Associated with the Induction of Different Neurotoxic Products which Together Determine Memory Impairments and Schizophrenia Symptom Dimensions. CNS Neurol Disord: Drug Targets. 2019;18(2):124–140. doi: 10.2174/1871527317666181119115532. [DOI] [PubMed] [Google Scholar]
- 30.Ivanovska M, Abdi Z, Murdjeva M, Macedo D, Maes A, Maes M. CCL-11 or Eotaxin-1: An Immune Marker for Ageing and Accelerated Ageing in Neuro-Psychiatric Disorders. Pharmaceuticals (Basel, Switzerland). 2020;13(9):230. doi: 10.3390/ph13090230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maes M, Rachayon M, Jirakran K, Sodsai P, Klinchanhom S, Gałecki P, et al. The Immune Profile of Major Dysmood Disorder: Proof of Concept and Mechanism Using the Precision Nomothetic Psychiatry Approach. Cells. 2022;11(7):1183. doi: 10.3390/cells11071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maes M, Carvalho AF. The Compensatory Immune-Regulatory Reflex System (CIRS) in Depression and Bipolar Disorder. Mol Neurobiol. 2018;55(12):8885–8903. doi: 10.1007/s12035-018-1016-x. [DOI] [PubMed] [Google Scholar]
- 33.Pipanmekaporn T, Wongpakaran N, Mueankwan S, Dendumrongkul P, Chittawatanarat K, Khongpheng N, et al. Validity and reliability of the Thai version of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Clin Interv Aging. 2014;9:879–885. doi: 10.2147/CIA.S62660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bangphichet A. Reliability and Validity of the Thai version of the Delirium Rating Scale-Revised-98 (DRS-R-98) 2008. [Google Scholar]
- 35.Cape E, Hall RJ, van Munster BC, de Vries A, Howie SE, Pearson A, et al. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1β in delirium after hip fracture. J Psychosom Res. 2014;77(3):219–225. doi: 10.1016/j.jpsychores.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark IA, Vissel B. The Inflammatory Nature of Post-surgical Delirium Predicts Benefit of Agents With Anti-TNF Effects Such as Dexmedetomidine. Front Neurosci. 2018;12:257. doi: 10.3389/fnins.2018.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22(4):189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber A, Wasiliew P, Kracht M. Interleukin-1beta (IL-1beta) processing pathway. Sci Signaling. 2010;3(105):cm2. doi: 10.1126/scisignal.3105cm2. [DOI] [PubMed] [Google Scholar]
- 39.Carvalho FA, Aitken JD, Gewirtz AT, Vijay-Kumar M. TLR5 activation induces secretory interleukin-1 receptor antagonist (sIL-1Ra) and reduces inflammasome-associated tissue damage. Mucosal Immunol. 2011;4(1):102–111. doi: 10.1038/mi.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Learn CA, Boger MS, Li L, McCall CE. The phosphatidylinositol 3-kinase pathway selectively controls sIL-1RA not interleukin-1beta production in the septic leukocytes. J Biol Chem. 2001;276(23):20234–20239. doi: 10.1074/jbc.M100316200. [DOI] [PubMed] [Google Scholar]
- 41.Ramírez-Pérez S, De la Cruz-Mosso U, Hernández-Bello J, Martínez-Bonilla GE, Ramírez-Dueñas MG, Pereira-Suárez AL, et al. High expression of interleukine-1 receptor antagonist in rheumatoid arthritis: Association with IL1RN*2/2 genotype. Autoimmunity. 2017;50(8):468–475. doi: 10.1080/08916934.2017.1412431. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernhard S, Hug S, Stratmann AEP, Erber M, Vidoni L, Knapp CL, et al. Interleukin 8 Elicits Rapid Physiological Changes in Neutrophils That Are Altered by Inflammatory Conditions. J Innate Immun. 2021;13(4):225–241. doi: 10.1159/000514885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leitão L, Alves CJ, Alencastre IS, Sousa DM, Neto E, Conceição F, et al. Bone marrow cell response after injury and during early stage of regeneration is independent of the tissue-of-injury in 2 injury models. FASEB J. 2019;33(1):857–872. doi: 10.1096/fj.201800610RR. [DOI] [PubMed] [Google Scholar]
- 45.Han AA, Currie HN, Loos MS, Scardoni G, Miller JV, Prince N, et al. The impact of cytokine responses in the intra- and extracellular signaling network of a traumatic injury. Cytokine. 2018;106:136–147. doi: 10.1016/j.cyto.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holbrook J, Lara-Reyna S, Jarosz-Griffiths H, McDermott M. Tumour necrosis factor signalling in health and disease. F1000Res. 2019;8:F1000. doi: 10.12688/f1000research.17023.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaheryar ZA, Khan MA, Adnan CS, Zaidi AA, Hänggi D, Muhammad S. Neuroinflammatory Triangle Presenting Novel Pharmacological Targets for Ischemic Brain Injury. Front Immunol. 2021;12:748663. doi: 10.3389/fimmu.2021.748663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aboumrad M, Shiner B, Riblet N, Mills PD, Watts BV. Factors contributing to cancer-related suicide: A study of root-cause analysis reports. Psychooncol. 2018;27(9):2237–2244. doi: 10.1002/pon.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kovtun A, Messerer DAC, Scharffetter-Kochanek K, Huber-Lang M, Ignatius A. Neutrophils in Tissue Trauma of the Skin, Bone, and Lung: Two Sides of the Same Coin. J Immunol Res. 2018;2018:8173983. doi: 10.1155/2018/8173983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bliźniewska-Kowalska K, Szewczyk B, Gałecka M, Su KP, Maes M, Szemraj J, et al. Is Interleukin 17 (IL-17) Expression A Common Point in the Pathogenesis of Depression and Obesity? J Clin Med. 2020;9(12):4018. doi: 10.3390/jcm9124018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Liu X, Zhong Y. Interleukin-17A: The Key Cytokine in Neurodegenerative Diseases. Front Aging Neurosci. 2020;12:566922. doi: 10.3389/fnagi.2020.566922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai SJ. Role of interleukin 8 in depression and other psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110173. doi: 10.1016/j.pnpbp.2020.110173. [DOI] [PubMed] [Google Scholar]
- 53.Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279(47):48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 54.Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vasunilashorn SM, Ngo LH, Inouye SK, Fong TG, Jones RN, Dillon ST, et al. Apolipoprotein E genotype and the association between C-reactive protein and postoperative delirium: Importance of gene-protein interactions. Alzheimer's Dementia. 2020;16(3):572–580. doi: 10.1016/j.jalz.2019.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plas M, Hemmer PHJ, Been LB, van Ginkel RJ, de Bock GH, van Leeuwen BL. Incidence and predictors of postoperative delirium after cytoreduction surgery-hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2018;117(2):260–268. doi: 10.1002/jso.24811. [DOI] [PubMed] [Google Scholar]
- 57.Knaak C, Vorderwülbecke G, Spies C, Piper SK, Hadzidiakos D, Borchers F, et al. C-reactive protein for risk prediction of post-operative delirium and post-operative neurocognitive disorder. Acta Anaesthesiol Scand. 2019;63(10):1282–1289. doi: 10.1111/aas.13441. [DOI] [PubMed] [Google Scholar]
- 58.Neerland BE, Hall RJ, Seljeflot I, Frihagen F, MacLullich AM, Raeder J, et al. Associations Between Delirium and Preoperative Cerebrospinal Fluid C-Reactive Protein, Interleukin-6, and Interleukin-6 Receptor in Individuals with Acute Hip Fracture. J Am Geriatr Soc. 2016;64(7):1456–1463. doi: 10.1111/jgs.14238. [DOI] [PubMed] [Google Scholar]
- 59.Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediators Inflamm. 2017;2017:3908061. doi: 10.1155/2017/3908061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang X, Zhang Y, Fan W, Tang W, Zhang C. Interleukin-17 Alteration in First-Episode Psychosis: A Meta-Analysis. Molecular neuropsychiatry. 2018;3(3):135–140. doi: 10.1159/000481661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maes M, Plaimas K, Suratanee A, Noto C, Kanchanatawan B. First Episode Psychosis and Schizophrenia Are Systemic Neuro-Immune Disorders Triggered by a Biotic Stimulus in Individuals with Reduced Immune Regulation and Neuroprotection. Cells. 2021;10(11):2929. doi: 10.3390/cells10112929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alsadany MA, Shehata HH, Mohamad MI, Mahfouz RG. Histone deacetylases enzyme, copper, and IL-8 levels in patients with Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2013;28(1):54–61. doi: 10.1177/1533317512467680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maes M, Bocchio Chiavetto L, Bignotti S, Battisa Tura GJ, Pioli R, Boin F, et al. Increased serum interleukin-8 and interleukin-10 in schizophrenic patients resistant to treatment with neuroleptics and the stimulatory effects of clozapine on serum leukemia inhibitory factor receptor. Schizophr Res. 2002;54(3):281–291. doi: 10.1016/S0920-9964(00)00094-3. [DOI] [PubMed] [Google Scholar]
- 64.Sherwood ER, Prough DS. Interleukin-8, neuroinflammation, and secondary brain injury. Crit Care Med. 2000;28(4):1221–1223. doi: 10.1097/00003246-200004000-00054. [DOI] [PubMed] [Google Scholar]
- 65.Pan W, Wu X, He Y, Hsuchou H, Huang EY, Mishra PK, et al. Brain interleukin-15 in neuroinflammation and behavior. Neurosci Biobehav Rev. 2013;37(2):184–192. doi: 10.1016/j.neubiorev.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laurent C, Deblois G, Clénet ML, Carmena Moratalla A, Farzam-Kia N, Girard M, et al. Interleukin-15 enhances proinflammatory T-cell responses in patients with MS and EAE. Neurol Neuroimmunol Neuroinflamm. 2021;8(1):e931. doi: 10.1212/NXI.0000000000000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi SX, Li YJ, Shi K, Wood K, Ducruet AF, Liu Q. IL (Interleukin)-15 Bridges Astrocyte-Microglia Crosstalk and Exacerbates Brain Injury Following Intracerebral Hemorrhage. Stroke. 2020;51(3):967–974. doi: 10.1161/STROKEAHA.119.028638. [DOI] [PubMed] [Google Scholar]
- 68.Fenimore J, Young HA. Regulation of IFN-γ Expression. Adv Exp Med Biol. 2016;941:1–19. doi: 10.1007/978-94-024-0921-5_1. [DOI] [PubMed] [Google Scholar]
- 69.Adamis D, Lunn M, Martin FC, Treloar A, Gregson N, Hamilton G, et al. Cytokines and IGF-I in delirious and non-delirious acutely ill older medical inpatients. Age Ageing. 2009;38(3):326–32. doi: 10.1093/ageing/afp014. [DOI] [PubMed] [Google Scholar]
- 70.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 71.Evans I, Dower SK, Francis SE, Crossman DC, Wilson HL. Action of intracellular IL-1Ra (Type 1) is independent of the IL-1 intracellular signalling pathway. Cytokine. 2006;33(5):274–280. doi: 10.1016/j.cyto.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 72.Westhoff D, Witlox J, Koenderman L, Kalisvaart KJ, de Jonghe JF, van Stijn MF, et al. Preoperative cerebrospinal fluid cytokine levels and the risk of postoperative delirium in elderly hip fracture patients. J Neuroinflammation. 2013;10:122. doi: 10.1186/1742-2094-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maes M, Song C, Yirmiya R. Targeting IL-1 in depression. Expert Opin Ther Targets. 2012;16(11):1097–1112. doi: 10.1517/14728222.2012.718331. [DOI] [PubMed] [Google Scholar]
- 74.Ul-Haq Z, Naz S, Mesaik MA. Interleukin-4 receptor signaling and its binding mechanism: A therapeutic insight from inhibitors tool box. Cytokine Growth Factor Rev. 2016;32:3–15. doi: 10.1016/j.cytogfr.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 75.Maes M, Sirivichayakul S, Matsumoto AK, Maes A, Michelin AP, de Oliveira SL, et al. Increased Levels of Plasma Tumor Necrosis Factor-α Mediate Schizophrenia Symptom Dimensions and Neurocognitive Impairments and Are Inversely Associated with Natural IgM Directed to Malondialdehyde and Paraoxonase 1 Activity. Mol Neurobiol. 2020;57(5):2333–2345. doi: 10.1007/s12035-020-01882-w. [DOI] [PubMed] [Google Scholar]
- 76.Noto MN, Maes M, Nunes SOV, Ota VK, Rossaneis AC, Verri WA, Jr, et al. Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur Neuropsychopharmacol. 2019;29(3):416–431. doi: 10.1016/j.euroneuro.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 77.Saraiva M, Vieira P, O'Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2020;217(1):e20190418. doi: 10.1084/jem.20190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180(9):5771–7. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 79.Maes M, Kanchanatawan B. In (deficit) schizophrenia, a general cognitive decline partly mediates the effects of neuro-immune and neuro-oxidative toxicity on the symptomatome and quality of life. CNS Spectr. 2021:1–10. 10.1017/S1092852921000419. [DOI] [PubMed]
- 80.Maes M, Vojdani A, Sirivichayakul S, Barbosa DS, Kanchanatawan B. Inflammatory and Oxidative Pathways Are New Drug Targets in Multiple Episode Schizophrenia and Leaky Gut, Klebsiella pneumoniae, and C1q Immune Complexes Are Additional Drug Targets in First Episode Schizophrenia. Mol Neurobiol. 2021;58(7):3319–3334. doi: 10.1007/s12035-021-02343-8. [DOI] [PubMed] [Google Scholar]
- 81.Vasupanrajit A, Jirakran K, Tunvirachaisakul C, Solmi M, Maes M. Inflammation and nitro-oxidative stress in current suicidal attempts and current suicidal ideation: a systematic review and meta-analysis. Mol Psychiatry. 2022;27(3):1350–1361. doi: 10.1038/s41380-021-01407-4. [DOI] [PubMed] [Google Scholar]
- 82.Scholtens RM, van Munster BC, Adamis D, de Jonghe A, Meagher DJ, de Rooij SE. Variability of Delirium Motor Subtype Scale-Defined Delirium Motor Subtypes in Elderly Adults with Hip Fracture: A Longitudinal Study. J Am Geriatr Soc. 2017;65(2):e45–e50. doi: 10.1111/jgs.14582. [DOI] [PubMed] [Google Scholar]
- 83.Wohleb ES, Godbout JP. Basic aspects of the immunology of neuroinflammation. Modern trends in pharmacopsychiatry. 2013;28:1–19. doi: 10.1159/000343964. [DOI] [PubMed] [Google Scholar]
- 84.Bennett FC, Molofsky AV. The immune system and psychiatric disease: a basic science perspective. Clin Exp Immunol. 2019;197(3):294–307. doi: 10.1111/cei.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21(6):727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012;36(2):764–785. doi: 10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Simons KS, van den Boogaard M, Hendriksen E, Gerretsen J, van der Hoeven JG, Pickkers P, et al. Temporal biomarker profiles and their association with ICU acquired delirium: a cohort study. Critical care (London, England) 2018;22(1):137. doi: 10.1186/s13054-018-2054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Majewski P, Zegan-Barańska M, Karolak I, Kaim K, Żukowski M, Kotfis K. Current Evidence Regarding Biomarkers Used to Aid Postoperative Delirium Diagnosis in the Field of Cardiac Surgery-Review. Medicina (Kaunas, Lithuania) 2020;56(10):493. doi: 10.3390/medicina56100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Faresjö T, Faresjö A. To match or not to match in epidemiological studies–same outcome but less power. Int J Environ Res Public Health. 2010;7(1):325–332. doi: 10.3390/ijerph7010325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brazauskas R, Logan BR. Observational Studies: Matching or Regression? Biol Blood Marrow Transplantation. 2016;22(3):557–563. doi: 10.1016/j.bbmt.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matching Beate Ritz B. R. EPI 200B Winter 2010 NOTE: many of the following slides are based on the lectures notes provided by Dr. Hal Morgenstern. 2010. [Google Scholar]
- 92.Lawton G. You're only as young as your immune system. New Scientist (1971) 2020;245(3275):44–8. doi: 10.1016/S0262-4079(20)30646-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Study flowchart.
Data Availability Statement
The dataset generated during and/or analyzed during the current study will be available from Prof. Dr. Michael Maes upon reasonable request and once the dataset has been fully exploited by the authors.