Abstract
The idea that myelination is driven by both intrinsic and extrinsic cues has gained much traction in recent years. Studies have demonstrated that myelination occurs in an intrinsic manner during early development and continues through adulthood in an activity-dependent manner called adaptive myelination. Motor learning, the gradual acquisition of a specific novel motor skill, promotes adaptive myelination in both the healthy and demyelinated central nervous system (CNS). On the other hand, exercise, a physical activity that involves planned, structured and repetitive bodily movements that expend energy and benefits one's fitness, promotes remyelination in pathology, but it is less clear whether it promotes adaptive myelination in healthy subjects. Studies on these topics have also investigated whether the timing of motor learning or physical exercise is important for successful addition of myelin. Here we review our current understanding of the relationship of motor skill learning and physical exercise on myelination.
Keywords: myelination, motor learning, exercise, oligodendrocytes, remyelination
Summary Statement
Studies reviewed herein show that motor learning promotes increased myelination in non-pathological situations in the adult and in remyelination, with critical windows existing for effectiveness. Physical exercise without skilled learning, however, may be restricted to promoting remyelination following demyelinating disease.
Introduction
Myelin, the lipid-rich membrane that coats axons, facilitates the conductance of action potentials, protects and metabolically supports underlying axons, and is required for proper motor and cognitive function in the nervous system (Bechler et al., 2018; Criste et al., 2014; Domènech-Estévez et al., 2015; Elazar et al., 2019; Saab and Nave, 2017). Myelin is damaged in multiple sclerosis, one of the most common neurological diseases in young adults (Compston & Coles, 2002), as well as in HIV, Alzheimer's disease, amyotrophic lateral sclerosis (ALS) and other neurodegenerative diseases (Jensen, Monnerie, et al., 2015; Jensen, Roth, et al., 2019; Kang et al., 2013), spinal cord injuries (SCI) (for review see Pukos et al., 2019), a variety of leukodystrophies (for review see Pouwels et al., 2014) and following perinatal white matter injury, often resulting in devastating disabilities (for review see Pouwels et al., 2014; and Van Tilborg et al., 2018). Myelination over development and life contributes to movement, motor learning and cognitive function by tuning axonal conduction velocity and syncronizing neuronal oscillatory activity – failure to adequately myelinate or form new myelin over life contributes to the phenotype of many of these disorders associated with myelin pathology (Gould et al., 2018; Kato et al., 2020; Pan et al., 2020; Steadman et al., 2020).
Much of our understanding of myelin has come from studies focusing on developmental myelination and on the maturation of the oligodendrocyte (OL), the cell that synthesizes myelin as part of its plasma membrane. Developmental myelination begins around birth where ample numbers of oligodendrocyte progenitor cells (OPCs) are present in germinal zones in the developing brain (Fu et al., 2002). These cells migrate to the paraenchyma and begin to mature (Yu et al, 1994). As OPCs mature, they acquire lipids and express characteristic myelin proteins, and extend processes to ensheath axons leaving intermittent spaces that organize the underlying axon into nodes of Ranvier, generating a complex molecular structure that leads to efficient propagation of action potentials (Baumann, & Pham-Dinh, 2001) Stages of the oligodendrocyte lineage can be identified by stage-specific external and internal markers. OPC express A2B5, NG2 and PDGFRα (Nishiyama et al., 1996; Raff et al., 1983). Immature oligodendrocytes express O4, 3’,5’ cyclic nucleotide phosphodiesterase (CNP), and GalC (Bansal et al., 1992; Hart et al., 1989; Nishiyama et al., 1996; Ranscht et al., 1982; Scherer et al., 1994). Mature oligodendrocytes express CNP and GalC, but also aspartoacylase (Madhavarao et al., 2004) and connexin47 and connexin32 (Menichella et al., 2003). Major myelin proteins are expressed in mature oligodendrocytes, and include myelin basic protein (MBP), proteolipid protein (PLP), myelin-associated glycoprotein (MAG), and myelin oligodendrocyte glycoprotein (MOG; Fulton et al., 2010; Grinspan, 2002; Solly et al., 1996). Myelin sheaths can be structurally characterized by thickness in a measurement known as “g-ratio”which measures the inner versus outer diameter of the myelin sheath. The thickness of the myelin affects the speed of conduction (West et al., 2016).
Until recently, it was unclear what controlled this developmental process. Studies showed that cues from neurons prompted myelination, therefore it was assumed that the process of myelination required neuronal input or at least extrinsic cues (Ishibashi et al., 2006; Stevens et al., 2002). Recent studies now show that OLs can myelinate non-neuronal targets (i.e., microfibers) in vitro and in the absence of external cues, thus indicating that intrinsic properties of OLs do play a significant role in myelination (Bechler et al. 2015; Lee, Chong, et al., 2013; Lee, Leach, et al., 2012). This intrinsic type of myelination is thought to be transcriptionally regulated and causes the formation of a basic number of sheaths of a characteristic size (Bechler et al., 2018). However, in vivo oligodendrocytes do not myelinate everything – in fact – there are inhibitory extrinsic cues, like Jam2, that prevent myelination of dendrites (Redmond et al., 2016).
Although textbook illustrations of myelin segments on axons suggest the continuity of the sheaths separated only by nodes of Ranvier in white matter regions, recent studies show that in the neocortex and subcortical areas, the amount of myelination is actually highly variable and long sections of unmyelinated axons exist (Olivares et al., 2001; Tomassy and Fossati, 2014). These unmyelinated segments are then available for myelination in the adult. The function of these discontinuously myelinated – predominant interneuron (Call and Bergles, 2021; Micheva et al., 2016) - axons is not yet known but could influence circuit connectivity.
How are the intrinsic and extrinsic cues that promote myelination distributed over the age span? We have so far discussed myelination during development, but this process continues into adulthood through an experience-dependent mechanism known as adaptive myelination in which new OLs are formed (Hill et al., 2018; Hughes et al., 2018; Mckenzie et al., 2014; Young et al., 2013) and new or existing OLs can potentially regulate the number and size of myelin sheaths based on local signals (McKenzie et al., 2014; Xiao et al., 2016; Young et al., 2013). Adaptive myelination is the augmentation of existing myelin sheaths to myelinated regions or addition of supplementary myelin. This is distinct from remyelination - which is the addition of myelin to demyelinated axons. In either case, myelination is primarily derived from the formation of new oligodendrocytes and does not depend directly on proliferation of OPCs. An OPC number is homeostatically maintained, so that if one OPC differentiates into an oligodendrocyte, an OPC will proliferate to replace it (Hughes et al., 2013). However, proliferation of OPCs alone does not necessarily result in increased myelin because the generated OPCs do not necessarily differentiate (Tang et al., 2000, Tikoo et al., 1998). In the studies covered in this review, we define increasing myelination with evidence of increase in numbers of mature oligodendrocytes, amount of myelin proteins, changes in myelin thickness or g-ratio, myelin or white matter on imaging, and not simply increase in OPC proliferation.
One of the processes that promotes adaptive myelination is motor learning, the gradual acquisition of a specific novel motor skill (Rösser et al., 2008) which can be tested experimentally in animal models with motor learning assays like complex running wheel training (McKenzie et al., 2014). Similarly, magnetic resonance imaging (MRI) has shown that humans who engaged in motor learning activities such as juggling or playing the piano show increases in white matter density in the brain (Bechler et al., 2018; Sampaio-Baptista et al., 2013; Scholz et al., 2009).
While motor learning involves training or instruction, physical exercise is more general and is classified as a physical activity that involves planned, structured and repetitive bodily movements that expend energy and benefits one's physical fitness (Caspersen et al., 1985). In this review, in addition to distinguishing between motor learning and physical exercise, we categorize exercise as either voluntary or forced. Voluntary exercise involves animals or humans exercising, as defined above, on their own terms. In mice this typically takes the form of voluntary wheel running (VWR) where an animal is placed in a cage with a regular running wheel and may run on the wheel as they please. Forced physical exercise, often treadmill running or swimming, can be defined as a structured physical task that a researcher imposes on a subject against one's will that results in energy expenditure through a series of repeated bodily movements (Bernardes, Brambilla, et al., 2016; Bernardes, Oliveira-Lima, et al., 2013; Chen et al., 2019; Deforges et al., 2009; Shahidi et al., 2020; Younsi et al., 2020). The effects of both motor learning and exercise appear to be time sensitive and critical windows exist for their effectiveness in adaptive myelination (Makinodan et al., 2012; Xiao et al., 2016). The studies to be reviewed have revealed that motor learning is more influential for adaptive myelination, while physical exercise is more influential for promoting remyelination during central nervous system (CNS) pathology.
This review will discuss: (a) current research regarding both motor skill learning and physical exercise as environmental cues for myelination and (b) critical windows for adaptive myelination, and (c) mechanisms that underlie adaptive myelination.
Adaptive Myelination is Promoted by Motor Learning
Adaptive myelination is characterized by changes in thickness, length, and/or number of myelin sheaths on an axon, changes in myelin protein expression and formation of new oligodendrocytes (Bechler et al., 2018; Mount and Monje, 2017). Novel motor learning can induce pre-existing oligodendrocytes to make new myelin sheaths, although it is unclear how often this occurs (Bacmeister et al., 2020). More commonly, novel motor tasks, such as the skilled reaching task (SR) or complex running wheel, can induce OPCs in rodents to proliferate, migrate, and differentiate into new oligodendrocytes, and these form new myelin sheaths (Bacmeister et al., 2020; McKenzie et al. 2014; Sampaio-Baptista et al., 2013; Xiao et al. 2016). In one study on adaptive myelination (Table 1), rats trained in SR for 15 min every day for 11 days had more MBP in the white matter contralateral to the trained paw then in rats performing unskilled reaching tasks (UR) and control rats, as well as higher white matter density in the external capsule, cingulum, corpus callosum on myelin-sensitive MRI sequences, together suggesting that myelin content had increased in white matter regions (Sampaio-Baptista et al., 2013). Additionally, McKenzie et al. (2014, Table 1) reported that 3 weeks of a complex wheel task in mice led to a significant increase in the number of mature OLs in the corpus callosum. OPCs increased after only 4–6 days of complex wheel running, and then immature and mature OLs increased by 40% in runners after 11 days on the complex wheel, and by 50% after 3 weeks (McKenzie et al., 2014).
Table 1.
Adaptive Myelination and Physical Activity.
| Activity type | Activity method | Duration | Subject | Findings | Timing of findings | Reference |
|---|---|---|---|---|---|---|
| Studies examining adaptive myelination | ||||||
| Motor learning | Reaching task | 15 min/day 11 days | Rats 4–5 months | MRI WM: Increased FA in the external capsule, cingulum, corpus callosum, and internal capsule contralateral to reaching paw | 11 days | Sampaio-Baptista et al. (2013) |
| Increased MBP concentration in areas contralateral to reaching paw | ||||||
| Motor learning | Finger tapping task | 10 min/day 4 weeks | Humans 18–40 years | MRI WM & GM:Increase in FA in the right hemisphere caudate nucleus and corticospinal tract, and tracts linking the middle frontal gyrus to the caudate nucleus | 4 weeks | Reid et al. (2017) |
| MRI GM: Decreased FA in nucleus accumbens | ||||||
| Motor learning | Juggling | 6 weeks | Humans 18–33 years | MRI WM: Significant FAincrease in in the right posterior intraparietal sulcus white matter | 6 weeks | Scholz et al. (2009) |
| Not statistically significant but notable decrease in previously observed increased FA | 10 weeks | |||||
| Motor learning | Running on a complex wheel | 3 weeks Mice were euthanized at various time points | Mice (P60 and P90) | WM: transient increase in OPCs in corpus callosum | 4–6 days | McKenzie et al. (2014) |
| WM: 40% increase in immature and mature OLs in the corpus callosum | 11 days | |||||
| WM: 94% of EdU+ cells were OL lineage cells in the corpus callosum | ||||||
| WM: 50% more mature OLs than controls in the corpus callosum | 3 weeks | |||||
| No increase in OPC proliferation when wheel was removed for a week and reintroduced | ||||||
| Motor learning | Lever-pulling task | 12 days | Mice (6 weeks) | GM: Increase in MBP mRNA expression in the left primary motor cortex | 12 days | Kato et al. (2019) |
| GM: More mature OLs in the left primary motor cortex in WT mice than in PLP-tg mice following motor learning | ||||||
| WM: More mature OLs in the subcortical white matter in WT mice than in PLP-tg mice following motor learning | ||||||
| Motor learning | Running on a complex wheel | 1 week Mice were euthanized at various time points | Mice P85 | WM: Significant increase in newly differentiating OLs in subcortical white matter | 2.5 h | Xiao et al. (2016) |
| WM & GM: Significant increase in newly differentiating OLs in the motor cortex and subcortical white matter | 4 hours | |||||
| WM & GM: 50% increase in newly differentiating OLs in the motor cortex and subcortical white matter | 12 h | |||||
| WM & GM: Two fold increase in newly differentiating OLs in the motor cortex and subcortical white matter | 24 h | |||||
| WM & GM: Significant increase in newly formed OLs in the motor cortex and subcortical white matter | 2–4 days | |||||
| WM & GM: Increase of newly differentiating OLs compared to earlier persisted | 8 days | |||||
| Voluntary physical exercise | Running on a regular wheel | 2 weeks | Mice 8 weeks | GM: Increase MBP expression in the motor cortex | 2 weeks | **Zheng et al. (2019) |
| GM: Significantly more OL lineage cells, OLs, and OPCs in the motor cortex | ||||||
| GM: No change in density PDGFRα+/Olig 2+ OPCs in the motor cortex | ||||||
| Voluntary physical exercise | Running on a regular wheel | 12 days Mice were euthanized at various time points | Mice P65 (adult) | WM: Significant increase in OPCs in the corpus callosum | 4 days | McKenzie et al. (2014) |
| Voluntary physical exercise | Running on a regular wheel | 125 days | Mice P21–P23 | GM: No increase in myelin in the cerebellum | 125 days | **Alvarez-Saavedra et al. (2016) |
| GM: Slight increase in OPC proliferation in the cerebellum | ||||||
| Voluntary physical exercise | Running on a regular wheel | 6 weeks | Mice 8 weeks | GM: No significant difference in MBP or CNP expression in the striatum | 6 weeks | **Mandolesi et al. (2019) |
| Voluntary physical exercise | Running on a regular wheel | 3, 7 or 28 days | Rats 2 months | No difference in the major components of myelin -- the four isoforms of MBP, CNP, or PLP/DM20 in the spinal cord | 3 days | Ghiani et al. (2007) |
| 1 week | ||||||
| 4 weeks | ||||||
| Voluntary physical exercise | Running on a regular wheel | 7 weeks | Mice 9 weeks | No increase in protein expression of MBP. 1.4-fold increase in PLP1 expression. Elevated RNA expression for MBP and PLP. No effect on CNP RNA expression. 1.4-fold increase in Myrf expression. No significant increase in the number of OPCs or OLs. All observations were made in the spinal cord. | 7 weeks | Yoon et al. (2016) |
| Voluntary physical exercise | Running on a regular wheel | 2 weeks | Rats | GM: Significant increase in OPCs in the frontal cortex but not in the retrosplenial cortex or occipital cortex | 2 weeks | Hall et al. (2014) |
| Forced exercise | Treadmill running | 3 weeks | Mice 4 weeks | WM: Increase in proliferating OPCs and mature OLs. Higher MBP intensity in the corpus callosum | 3 weeks | Chen et al. (2019) |
| Voluntary physical exercise | Running on a regular wheel | 2 weeks | Mice 8–12 weeks | GM: 1.2 fold increase in number of OPCs WM: No OPC proliferation or differentiation observed in the corpus callosum GM: No OPC proliferation or differentiation observed in the piriform cortex | 2 weeks | Eugenin von Bernhardi and Dimou (2022) |
| 4 weeks | GM: significant increase differentiation of GPR17+ OPC cells GM: 1.45 fold increase proliferation of GPR17+ OPC glia GM: 1.4 fold increase in number of OPCs GM: 36.6% increase in OPC proliferation 130.2% increase in NG2 cell differentiation WM: No OPC proliferation or differentiation observed in the corpus callosum GM: No OPC proliferation or differentiation observed in the piriform cortex | 4 weeks | ||||
(**) indicates that although a voluntary exercise paradigm was used, the experiment also used motor learning assays to assess motor ability. It is indicated whether changes in OL lineage cells occurred in white matter (WM) or grey matter (GM). Acronyms: fractional anisotropy (FA), oligodendrocytes (OL), oligodendrocyte precursors cells (OPC).
McKenzie et al. (2014) demonstrated that not only can motor learning promote adaptive myelination, but their data also suggest that formation of new oligodendrocytes and myelin sheaths is required for motor learning and skill acquisition. Transgenic mice unable to generate new oligodendrocytes due to a conditional deletion in Myrf - a transcription factor required to initiate myelination in oligodendrocytes – had slower running speeds and worse performance on the complex wheel task compared to controls. These findings were further corroborated by Kato et al. (2019, Table 1). WT mice or transgenic mice with extra copies of the Plp1 gene (PLP-tg) - leading to abnormal (thinner) myelin sheaths and reduced conduction velocity in myelinated CNS tracts - were trained for one hour a day for twelve days on a right forelimb-dependent voluntary lever pulling task. After 12 days of training, more mature OLs formed with higher MBP mRNA in the left primary motor cortex and subcortical white matter in trained WT mice compared to trained PLP-tg mice (Kato et al., 2019). Unlike WT mice, PLP-tg mice failed to improve conduction velocity and input synchronization after a motor task, providing evidence that adaptive myelination alters circuit function in motor learning.
Studies on motor learning and myelination have also been conducted in human subjects. Motor learning with motor sequence tasks and juggling increases white matter density in human brains, as indicated by MRI scans (Marins et al., 2019; Reid et al., 2017; Scholz et al., 2009, Table 1). Reid et al. (2017, Table 1) trained 24 right-handed human participants to successively touch their left thumb to the other fingers on their left hand. Participants practiced the motor sequence task for 10 min daily over the span of four weeks. Fractional anisotropy (FA) diffusion MRI - acquired before and after motor training - revealed increased white matter density following motor training in the right caudate nucleus, corticospinal tract, and tracts linking the middle frontal gyrus to the caudate nucleus. There was also a bilateral decrease in FA in the nucleus accumbens (Reid et al., 2017). The authors cautiously suggest that increases in white matter density reflect increases in actual myelin but changes in numbers of oligodendrocytes or amount of myelin were not measured. The results of this study remain intruiging but further research is needed to directly show the relationship between increase in white matter density on MRI and any corresponding changes in cellular or myelin content performed.
Critical Windows for Adaptive Myelination
Several studies have examined the timing and persistence of adaptive myelination following motor learning. Consistent with the McKenzie et al. (2014) study, Xiao et al. (2016, Table 1) demonstrated that more oligodendrocytes (OLs) differentiated when mice were presented with a novel motor learning task on a complex wheel, but this occurred within a rapid time frame. Differentiating OLs increased in subcortical white matter as early as 2.5 h into training and after 4 h in the motor cortex. The increase of newly differentiating OLs was most prominent during the first 24 h of training, and continued at a lower rate 8 days following the introduction of the complex wheel. As such, this study suggests that the first 24 h of training a novel motor learning task may be a critical window to promote adaptive myelination (Xiao et al., 2016). This finding also seems to indicate that initial exposure to a training task potentially has the most significant effect on the rate of OL differentiation. However, many studies have shown that the differentiation from OPC to mature myelinating OL takes atleast 48–72 h, so it is unclear whether the rapid appearance of new OLs and increased myelin represents the stabilization of cells already in the process of differentiating and forming new myelin - that would normally not be maintained – or the novel differentiation of OPCs. Future research is needed to identify the cellular and molecular mechanisms that control this rapid generation.
McKenzie et al. (2014) demonstrated that after a month without training on the previously learned motor task (running on a complex wheel), mice that underwent adaptive myelination had maintained the ability to outperform mice who did not undergo adaptive myelination when reintroduced to the complex wheel. Although the improved task performance persisted, they did not determine whether white matter architecture created through adaptive myelination remains stable over time. It has been previously demonstrated in humans and mice that OLs formed through developmental myelination are stable over the life-span with little turnover (Hill et al., 2018; Hughes et al., 2018; Yeung et al., 2014). However, whether or not this is also true for OLs developed through adaptive myelination during adulthood requires further investigation.
Additionally, adaptive myelination during motor learning appears to occur only when the the motor task is novel. When reintroduced to the task following a 1–2 week resting period, experienced mice showed no additional increase in OPC proliferation of differentiation. The above findings suggest that adaptive myelination is restricted to novel learning experiences (McKenzie et al., 2014; Xiao et al., 2016). It is clear from the literature that augmentation to existing myelin sheaths not only is possible well into adulthood, but also plays an important role in motor skill learning. The findings across studies consistently demonstrate that motor learning promotes adaptive myelination in both humans and animals. Additionally, the first 24 h following exposure to novel motor tasks may represent a critical window for adaptive myelination.
Voluntary Exercise Does Not Necessarily Promote Adaptive Myelination
The impact of physical exercise, as opposed to motor learning, on adaptive myelination is more ambiguous. Several studies show increased OPC proliferation in the brain due to physical exercise, but those studies are at odds as to which brain regions are affected. For example, Hall et al. (2014, Table 1) found that adult male rats that ran voluntarily on a wheel for 14 days had significantly more OPCs in the frontal cortex but not the retrosplenial cortex or occipital cortex. In an additional experiment in the McKenzie et al. (2014) study discussed above looked at VWR running instead of running on a complex wheel, 4 days of VWR led to increased numbers of OPCs in the corpus callosum. However, in both of these studies, the increase in OPCs in response to VWR did not result in increased numbers of OLs or myelin proteins, therefore these studies are not indicative of adaptive myelination.
Additional studies also did not find a relationship between physical exercise and adaptive myelination. Alvarez-Saavedra et al. (2016, Table 1) found that mice engaged in voluntary physical exercise showed no increase in myelin, as observed using transmission electron microscopy (TEM) 125 days post-VWR. In running mice as compared to sedentary mice, there was a slight, but not statistically significant, increase in OPC number in the cerebellum without an increase in the number of OLs in either the cerebellum or brain stem. Of note, the mice in the Alvarez-Saavedra et al. (2016) study were also trained on the rotarod assay prior to VWR assessment, so motor learning may also have played a role. Similarly, Mandolesi et al. (2019, Table 1), provided mice with a running wheel to run on voluntarily for 6 weeks (physical exercise) and performed a grip strength task at weeks three and five and a rotarod test at week five. No significant difference in expression of MBP or CNP was found between the sedentary and VWR mice following the 6-week period in the corpus callosum and striatum, and no changes were observed in numbers of OL lineage cells in the corpus callosum, similar to Zheng et al. (2019). Together, these findings show that motor tasks in conjunction with voluntary exercise have differing effects on OL lineage cells dynamics.
It is possible that voluntary exercise promotes adaptive myelination at a different rate than motor learning, and only in some brain regions. Eugenin Von Bernhardi and Dimou (2022) found that VWR after both 2 weeks and 4 weeks led to an increase in newly generated OLs in the motor cortex, but not the corpus callosum or piriform cortex. Notably, 4 weeks of VWR induced production of more OLs relative to 2 weeks, which were mainly derived from OPCs directly differentiating into mature OLs,(62.30%) as opposed to OPCs that first proliferate prior to differentiating (62.3% v. 37.7%, respectively). They also found that a subset of OPCs, those that expressed the G-protein coupled receptor 17 (GPR17), showed a delayed differentiation response to VWR. In this population of cells, 6 weeks of VWR were necessary to induce an increase in amount of newly differentiated oligodendrocytes derived from GPR17+ OPCs (Eugenin von Bernhardi and Dimou 2022). Together, these results demonstrate that exercise can induce heterogeneous adaptive myelination, and varies by on brain region, “dose” of exercise defined by amount of exercise over time, and type of OPC, defined by specific protein expression profile.
Similar to the Von Bernhardi and Dimou study, Zheng et al., (2019, Table 1) found that that two weeks of VWR in adult 8-week old mice induced more OLs and MBP expression in the motor cortex, but no change in the corpus callosum (in contrast to McKenzie et al., (2014)). Notably, in addition to VWR, the mice used in this study had been previously trained on the rotarod and beam walking tests (Zheng et al., 2019), so adaptive myelination was probably not entirely based on physical exercise alone. Given that adaptive myelination may initiate as rapidly as 2.5 h following the introduction to a motor learning task (Xiao et al. 2016), the combination of a motor learning task introduction followed by a period of voluntary exercise, promotes increased myelination.
In the spinal cord, two studies had somewhat conflicting findings regarding adaptive myelination following voluntary exercise (Ghiani et al., 2007; Yoon et al., 2016, Table 1). Ghiani et al. (2007) gave 2 month old rats access to a regular running wheel to run on voluntarily for either 3, 7 or 28 days. There were no differences in the major components of myelin - MBP, CNP, or DM20 – at 3, 7, or 28 days post-VWR in the lumbar spinal cord. While Yoon et al. (2016) also found that 9 week old mice did not increase MBP or CNP protein in the spinal cord after 7 weeks of VWR compared to mice that were sedentary for 7 weeks, PLP1 protein increased by 140%, and MBP, PLP and Myrf RNA increased. However, there was no significant increase in the number of OPCs or OLs in the observed area of the spinal cord (Yoon et al., 2016). While it is possible that physical exercise altered the metabolism of the OL lineage cells, number of myelin sheaths, or features of individual myelin sheaths already present, neither study found a difference in number of OL lineage cells in mice undergoing voluntary exercise.
While there is disagreement in the current literature regarding the relationship between voluntary exercise and adaptive myelination, the studies discussed suggest that physical activity promotes adaptive myelination in the motor cortex by inducing formation of more oligodendrocytes (Eugenin Von Bernhardi and Dimou 2022; Zheng et al., 2019) or modifying myelin itself in the spinal cord (Yoon, et al., 2016). It is possible that exposure to a motor learning task (even for a single time), can influence OPC proliferation and myelination while physical activity alone may induce changes in myelination on a slower time course. In order to more accurately assess the impact of voluntary exercise on myelination, future studies should refrain from including additional motor learning assays or tasks when measuring the effects of voluntary exercise to ensure that the proper variable is isolated. At this point, based on the current research, it remains unclear whether voluntary exercise alone promotes adaptive myelination in the CNS, and whether formation of new OLs is required (vs. modifying existing myelin).
Forced Exercise Can Promote Adaptive Myelination
At least one study has looked at the effect of forced physical activity on adaptive myelination in young mice. Chen et al. (2019, Table 1) forced 4 week old mice to run on a treadmill for 1 h per day for 3 weeks. Following the exercise paradigm, more proliferating OPCs and mature OLs and higher MBP intensity were detected in the corpus callosum; myelin in these region had increased thickness as measured by electron microscopy, suggesting that forced physical exercise promoted adaptive myelination in the corpus callosum. More studies are needed to confirm this finding.
Adaptive Myelination is Distinct From Remyelination
Adaptive myelination differs from remyelination in several ways. First, as previously discussed, adaptive myelination occurs in healthy brains while remyelination occurs in brains with pathology. While exercise does not necessarily promote adaptive myelination in healthy brains, the role of voluntary exercise on CNS demyelinating pathology could be multi-faceted, as studies have reported that voluntary exercise can prevent demyelination or promote remyelination (Alvarez-Saavedra et al., 2016; Jensen et al. 2018; Jiang et al., 2017; Mandolesi et al., 2019; Pryor et al., 2015, Siegenthaler et al., 2008).
Mandolesi et al. (2019, Table 2) tested the effect of voluntary exercise in the cuprizone toxin model of demyelination. Voluntary exercise began on a regular running wheel at the onset of cuprizone demyelination and continued throughout the 6-week feeding period until the mice were euthanized. In cuprizone treated mice, physical exercise resulted in relatively more MBP and CNP in the corpus callosum, cingulum and striatum after 3 weeks and persisting up to 6 weeks, compared to cuprizone-treated sedentary mice. Since remyelination begins spontaneously in the corpus callosum during cuprizone feeding (Mandolesi et al., 2019), it was not clear whether voluntary exercise prevented loss of oligodendrocytes and myelin or promoted formation of new oligodendrocytes and/or myelin sheaths.
Table 2.
Remyelination and Physical Activity.
| Activity type | Activity method | Duration | Disease model and subject | Findings | Timing of findings | Reference |
|---|---|---|---|---|---|---|
| Studies examining remyelination | ||||||
| Voluntary exercise | Running on a regular wheel | 28 days Mice were sacrificed at various time points | lysolecithin mouse model 8–12 weeks old | Observed OPC proliferation in the spinal cord | 3–5 days | Jensen et al., (2018) |
| Observed OPC differentiation in the spinal cord | 5–14 days | |||||
| 7 days of running followed by a 7 day rest prior to demyelination | lysolecithin mouse model 8–12 weeks old | Remyelination was observed in the spinal cord | 10–28 days | |||
| 37% increase in OL lineage cells in the spinal cord | 14 DPL | |||||
| Voluntary exercise | Running on a regular wheel | 6 weeks | Cuprizone mouse model 8 weeks old | WM & GM: Partial recovery of MBP and CNP in the corpus callosum, cingulum and striatum | 3–6 weeks | **Mandolesi et al. (2019) |
| Motor learning | Reaching task | 1 week | Cuprizone mouse model 6–8 weeks old | GM: More OLs and myelin sheaths generated in the motor cortex compared to untrained mice following cuprizone treatment | 7 weeks | Bacmeister et al. (2020) |
| GM: some surviving OLs could make new myelin sheaths during motor learning | ||||||
(**) indicates that although a voluntary exercise paradigm was used, the experiment also used motor learning assays to assess motor ability. It is indicated whether changes in OL lineage cells occurred in white matter (WM) or grey matter (GM).
A 2018 study by Jensen and colleagues (Table 2) used a different toxin-induced model of demyelination, lysolecithin-induced spinal cord demyelination, and observed the effects of unlimited access to a running wheel during the remyelination period. In this model, OPCs proliferate at 3–5 days post lesion (DPL), and differentiate into OLs between 5–14 DPL. VWR induced more OPCs in lesions at 3–4 DPL (up to 34% more), with 48% more MBP and 2.11-fold increase in myelinated axon density between 5–14 DPL, but a similar rate of formation of new OL, compared to sedentary mice. They also found that VWR novelty did not affect the rate of OPC proliferation and differentiation during the recovery period. Post-lysolecithin-treated mice were permitted to run on a regular wheel voluntarily for 7 days, followed by a 7-day rest period. Increases in OPC proliferation and differentiation continued when mice were reintroduced to the wheel following the rest period, unlike McKenzie et al. (2014) and Xiao et al. (2016), suggesting that the cues from exercise promoting remyelination are distinct from adaptive myelination. Exercise also appears to promote myelination in various disease models where myelin loss is an indirect consequence of the condition itself such as spinal cord injury (Siegenthaler et al., 2008).
While motor learning has been shown to be effective at promoting both adaptive myelination and remyelination, the underlying mechanisms may differ. Bacmeister et al. (2020, Table 2) used longitudinal two-photon in vivo imaging and tracking of individual OL and myelin sheaths to directly observe oligodendrocyte and myelin dynamics. This group showed that mice introduced to motor learning (reaching task) 10 days post-cuprizone had a higher density of both OLs and myelin sheaths in the motor cortex compared to untrained mice (Bacmeister et al., 2020). Although generation of new OLs likely accounts for the bulk of new myelin sheaths generated during the recovery period, Bacmeister et al. (2020) also found examples of pre-existing OLs that formed new myelin sheaths on demyelinated axons during the motor learning period (Bacmeister et al. 2020). In contrast, they did not find examples of pre-existing oligodendrocytes forming new myelin sheaths in response to motor learning alone in healthy brains. A new study in zebrafish also found pre-existing oligodendrocytes forming new myelin sheaths after injury, although these myelin sheaths formed close to the oligodendrocyte cell body and may ensheath neuronal cell bodies (Neely et al., 2022). Together, these studies suggest that the processes that underly remyelination and adaptive myelination differ. Additional research is needed to further elucidate the differences in the cellular mechanisms that promote formation of new oligodendrocytes, or new myelin sheaths from pre-existing OL, in adaptive myelination and remyelination.
Mechanisms Underlying Adaptive Myelination
Although potential mechanisms underlying adaptive myelination have been reviewed elsewhere (de Faria et al., 2021; Habermacher et al., 2019; Monje 2018; Mount & Monje 2017; Tomlinson et al., 2016), only a few of of the studies covered in this review identified specific mechanisms that link motor learning or exercise to adaptive myelination. For example, Zheng et al. (2019) found that voluntary wheel running decreased motor cortex expression of components of the Wnt/Beta-catenin pathway, a pathway known to inhibit myelination when activated (Feigenson et al., 2009). Chen et al., (2019), found that adaptive myelination was blocked after forced exercise if mice were treated with rapamycin, an inhibitor of the mTOR pathway. Neuronal activity itself induces adaptive myelination in vivo via release of neurotransmitters such as glutamate and GABA that can be sensed by OPCs (Gibson et al., 2014, Wake et al., 2011, 2015). However, it is not clear what specific cues instruct oligodendrocytes to myelinate multiple inputs to a single circruit (as suggested by the Kato et al., 2020 study). Astrocytes secrete growth factors like BDNF, which induces myelination through activation of the the TrkB receptor, and likely also promotes adaptive myelination (Geraghty et al., 2019, Saitta et al., 2021). Clearly, more research is necessary to identify mechanisms, and complex coordination between oligodendrocyte lineage cells, neurons, and astrocytes, to clarify the processes that promote adaptive myelination and remyelination driven by exercise and motor learning.
Conclusion
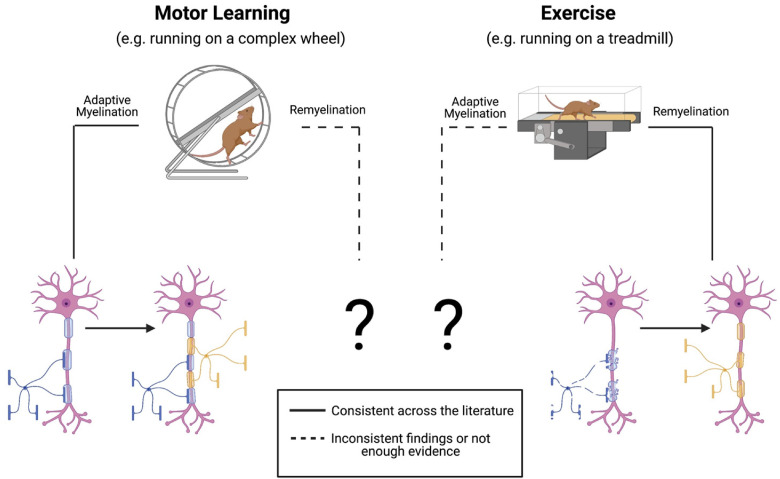
Our understanding of the cues for myelination in developing and adult CNS is continually expanding. It is now clear that motor learning plays a critical role in adaptive myelination. Studies show that despite the variability in age tested, type of intervention and brain location looked at, that some type of motor intervention can affect OL lineage and myelin dynamics, although the role of physical exercise alone still needs to be better understood (Figure 1). Additionally, extrinsic cues such as physical exercise and motor learning are effective in promoting remyelination, although the current literature suggests that the mechanisms underlying adaptive myelination and remyelination are distinct (Figure 1). Finally, the specific cues that promote OL lineage changes and formation of myelin need to be identified.
Figure 1.
Motor learning promotes adaptive myelination and both exercise and motor learning promote remyelination. However, it is not clear whether exercise alone promotes adaptive myelination, and whether motor learning promotes remyelination outside of the motor cortex or in multiple demyelinating pathologies.
The discovery that myelination continues into adulthood in an experience-dependent manner provides an exciting new avenue for future research that focuses on learning, and whether one type of learning affects myelination in one or more brain regions. It also underscores the need to consider the importance of adaptive myelination in acquiring new skills throughout the age span and how this might be compromised in diseases that disrupt myelin. The identification of the ability of both exercise, voluntary or forced, and motor learning to encourage remyelination suggests new therapeutic avenues as well for inherited and acquired disorders of myelin.
Open Questions and Future Directions
The literature clearly demonstrates a difference in OL lineage cell response to motor learning as compared to physical exercise. An open question to be explored will be the specific molecular mechanisms that cause adaptive myelination in motor learning and physical exercise and whether they are distinct from one another. Additionally, future studies should aim to investigate whether heterogeneity in the oligodendrocyte lineage, either intrinsic OPC variations or variation in location, affects adaptive myelination. This is an exciting new avenue of research that can be taken in many directions in future studies.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Mental Health, (grant number 1 R01 MH 098742, 1R01MH126773).
ORCID iD: Judith B. Grinspan https://orcid.org/0000-0003-0940-5314
References
- Alvarez-Saavedra M., De Repentigny Y., Yang D., O’Meara R. W., Yan K., Hashem L. E., Racacho L., Ioshikhes I., Bulman D. E., Parks R. J., Kothary R., Picketts D. J. (2016). Voluntary running triggers VGF-mediated oligodendrogenesis to prolong the lifespan of Snf2h-null ataxic mice. Cell Reports, 17(3), 862–875. 10.1016/j.celrep.2016.09.030 [DOI] [PubMed] [Google Scholar]
- Bacmeister C. M., Barr H. J., McClain C. R., Thornton M. A., Nettles D., Welle C. G., Hughes E. G. (2020). Motor learning promotes remyelination via new and surviving oligodendrocytes. Nature Neuroscience, 23(7), 819–831. 10.1038/s41593-020-0637-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R., Stefansson K., Pfeiffer S. E. (1992). Proligodendroblast antigen (POA), a developmental antigen expressed by A007/O4-positive oligodendrocyte progenitors prior to the appearance of sulfatide and galactocerebroside. Journal of Neurochemistry, 58(6), 2221–2229. 10.1111/j.1471-4159.1992.tb10967.x [DOI] [PubMed] [Google Scholar]
- Baumann N., Pham-Dinh D. (2001). Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiological Reviews, 81(2), 871–927. 10.1152/physrev.2001.81.2.871 [DOI] [PubMed] [Google Scholar]
- Bechler M. E., Byrne L., Ffrench-Constant C. (2015). CNS Myelin Sheath lengths are an intrinsic property of oligodendrocytes. Current Biology, 25(18), 2411–2416. 10.1016/j.cub.2015.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechler M. E., Ffrench-Constant C. (2018). Intrinsic and adaptive myelination-A sequential mechanism for smart wiring in the brain. Dev Neurobiol, 78, 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechler M. E., Swire M., Ffrench-Constant C. (2018). Intrinsic and adaptive myelination-A sequential mechanism for smart wiring in the brain. Developmental Neurobiology, 78(2), 68–79. 10.1002/dneu.22518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardes D., Brambilla R., Bracchi-Ricard V., Karmally S., Dellarole A., Carvalho-Tavares J., Bethea J. R. (2016). Prior regular exercise improves clinical outcome and reduces demyelination and axonal injury in experimental autoimmune encephalomyelitis. Journal of Neurochemistry, 136(Suppl 1), 63–73. 10.1111/jnc.13354 [DOI] [PubMed] [Google Scholar]
- Bernardes D., Oliveira-Lima O. C., Silva T. V., Faraco C. C., Leite H. R., Juliano M. A., Santos D. M., Bethea J. R., Brambilla R., Orian J. M., Arantes R. M., Carvalho-Tavares J. (2013). Differential brain and spinal cord cytokine and BDNF levels in experimental autoimmune encephalomyelitis are modulated by prior and regular exercise. Journal of Neuroimmunology, 264(1-2), 24–34. 10.1016/j.jneuroim.2013.08.014 [DOI] [PubMed] [Google Scholar]
- Call C. L., Bergles D. E. (2021). Cortical neurons exhibit diverse myelination patterns that scale between mouse brain regions and regenerate after demyelination. Nature Communications, 12(1-2), 4767. 10.1038/s41467-021-25035-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen C. J., Powell K. E., Christenson G. M. (1985). Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Reports, 100, 126–131. [PMC free article] [PubMed] [Google Scholar]
- Chen K., Zheng Y., Wei J. A., Ouyang H., Huang X., Zhang F., Lai C. S. W., Ren C., So K. F., Zhang L. (2019). Exercise training improves motor skill learning via selective activation of mTOR. Science Advances, 5(7), eaaw1888. 10.1126/sciadv.aaw1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A., Coles A. (2002). Multiple sclerosis. Lancet (London, England), 359(9313), 1221–1231. 10.1016/S0140-6736(02)08220-X [DOI] [PubMed] [Google Scholar]
- Criste G., Trapp B., Dutta R. (2014). Axonal loss in multiple sclerosis: Causes and mechanisms. Handbook of Clinical Neurology, 122, 101–113. 10.1016/B978-0-444-52001-2.00005-4 [DOI] [PubMed] [Google Scholar]
- de Faria O., Jr., Pivonkova H., Varga B., Timmler S., Evans K. A., Karadottir R. T. (2021) Periods of synchronized myelin changes shape brain function and plasticity. Nature Neuroscience 24(11), 1508–1521. 10.1038/s41593-021-00917-2 [DOI] [PubMed] [Google Scholar]
- Deforges S., Branchu J., Biondi O., Grondard C., Pariset C., Lecolle S., Lopes P., Vidal P. P., Chanoine C., Charbonnier F. (2009). Motoneuron survival is promoted by specific exercise in a mouse model of amyotrophic lateral sclerosis. Journal of Physiology , 587(14), 3561–3572. 10.1113/jphysiol.2009.169748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech-Estevez E., Baloui H., Repond C., Rosafio K., Medard J. J., Tricaud N., Pellerin L., Chrast R. (2015). Distribution of monocarboxylate transporters in the peripheral nervous system suggests putative roles in lactate shuttling and myelination. Journal of Neuroscience, 35(10), 4151–4156. 10.1523/JNEUROSCI.3534-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazar N., Vainshtein A., Rechav K., Tsoory M., Eshed-Eisenbach Y., Peles E. (2019). Coordinated internodal and paranodal adhesion controls accurate myelination by oligodendrocytes. Journal of Cell Biology, 218(9), 2887–2895. 10.1083/jcb.201906099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin von Bernhardi J., Dimou L. (2022). Oligodendrogenesis is a key process for cognitive performance improvement induced by voluntary physical activity. Glia, 70(6), 1052–1067. Epub 2022 Feb 1. PMID: 35104015. 10.1002/glia.24155 [DOI] [PubMed] [Google Scholar]
- Feigenson K., Reid M., See J., Crenshaw E. B., 3rd, Grinspan J. B. (2009). Wnt signaling is sufficient to perturb oligodendrocyte maturation. Molecular and Cellular Neuroscience, 42(3), 255–265. 10.1016/j.mcn.2009.07.010 [DOI] [PubMed] [Google Scholar]
- Fu H., Qi Y., Tan M., Cai J., Takebayashi H., Nakafuku M., Richardson W., Qiu M. (2002). Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2.2 in the control of oligodendrocyte differentiation. Development (Cambridge, England), 129(3), 681–693. 10.1242/dev.129.3.681 [DOI] [PubMed] [Google Scholar]
- Fulton D., Paez P. M., Campagnoni A. T. (2010). The multiple roles of myelin protein genes during the development of the oligodendrocyte. ASN Neuro, 2(1), e00027. 10.1042/AN20090051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty A. C., Gibson E. M., Ghanem R. A., Greene J. J., Ocampo A., Goldstein A. K., Ni L., Yang T., Marton R. M., Pasca S. P., Greenberg M. E., Longo F. M., Monje M. (2019). Loss of adaptive myelination contributes to methotrexate chemotherapy-related cognitive impairment. Neuron, 103(2), 250–265.e8. 10.1016/j.neuron.2019.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani C. A., Ying Z., de Vellis J., Gomez-Pinilla F. (2007). Exercise decreases myelin-associated glycoprotein expression in the spinal cord and positively modulates neuronal growth. Glia, 55(9), 966–975. 10.1002/glia.20521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson E. M., Purger D., Mount C. W., Goldstein A. K., Lin G. L., Wood L. S., Inema I., Miller S. E., Bieri G., Zuchero J. B., Barres B. A., Woo P. J., Vogel H., Monje M. (2014). Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science (New York, N.Y.), 344(6183), 1252304. 10.1126/science.1252304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. A., Busquet N., Shepherd D., Dietz R. M., Herson P. S., Simoes De Souza F. M., Li A., George N. M., Restrepo D., Macklin W. B. (2018). Mild myelin disruption elicits early alteration in behavior and proliferation in the subventricular zone. Elife, 7, e34783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspan J. (2002). Cells and signaling in oligodendrocyte development. Journal of Neuropathology & Experimental Neurology, 61(4), 297–306. 10.1093/jnen/61.4.297 [DOI] [PubMed] [Google Scholar]
- Habermacher C., Angulo M. C., Benamer N. (2019). Glutamate versus GABA in neuron-oligodendroglia communication. Glia, 67(11), 2092–2106. 10.1002/glia.23618 [DOI] [PubMed] [Google Scholar]
- Hall J. M., Vetreno R. P., Savage L. M. (2014). Differential cortical neurotrophin and cytogenetic adaptation after voluntary exercise in normal and amnestic rats. Neuroscience, 258, 131–146. 10.1016/j.neuroscience.2013.10.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart I. K., Richardson W. D., Bolsover S. R., Raff M. C. (1989). PDGF And intracellular signaling in the timing of oligodendrocyte differentiation. Journal of Cell Biology, 109(6), 3411–3417. 10.1083/jcb.109.6.3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. A., Li A. M., Grutzendler J. (2018). Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nature Neuroscience, 21(5), 683–695. 10.1038/s41593-018-0120-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes E. G., Kang S. H., Fujaya M., Bergles D. E. (2013). Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nature Neuroscience, 16(6), 668–676. 10.1038/nn.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes E. G., Orthmann-Murphy J. L., Langseth A. J., Bergles D. E. (2018). Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nature Neuroscience, 21(5), 696–706. 10.1038/s41593-018-0121-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T., Dakin K. A., Stevens B., Lee P. R., Kozlov S. V., Stewart C. L., Fields R. D. (2006). Astrocytes promote myelination in response to electrical impulses. Neuron, 49(6), 823–832. 10.1016/j.neuron.2006.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B. K., Monnerie H., Mannell M. V., Gannon P. J., Espinoza C. A., Erickson M. A., Bruce-Keller A. J., Gelman B. B., Briand L. A., Pierce R. C., Jordan-Sciutto K. L., Grinspan J. B. (2015). Altered oligodendrocyte maturation and myelin maintenance: the role of antiretrovirals in HIV-associated neurocognitive disorders. Journal of Neuropathology & Experimental Neurology, 74(11), 1093–1118. 10.1097/NEN.0000000000000255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B. K., Roth L. M., Grinspan J. B., Jordan-Sciutto K. L. (2019). White matter loss and oligodendrocyte dysfunction in HIV: A consequence of the infection, the antiretroviral therapy or both? Brain Research, 1724, 146397. 10.1016/j.brainres.2019.146397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. K., Michaels N. J., Ilyntskyy S., Keough M. B., Kovalchuk O., Yong V. W. (2018). Multimodal enhancement of remyelination by exercise with a pivotal role for oligodendroglial PGC1alpha. Cell Reports, 24(12), 3167–3179. 10.1016/j.celrep.2018.08.060 [DOI] [PubMed] [Google Scholar]
- Jiang T., Zhang L., Pan X., Zheng H., Chen X., Li L., Luo J., Hu X. (2017). Physical exercise improves cognitive function together with microglia phenotype modulation and remyelination in chronic cerebral hypoperfusion. Frontiers in Cellular Neuroscience, 11, 404. 10.3389/fncel.2017.00404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. H., Li Y., Fukaya M., Lorenzini I., Cleveland D. W., Ostrow L. W., Rothstein J. D., Bergles D. E. (2013). Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nature Neuroscience, 16(5), 571–579. 10.1038/nn.3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato D., Wake H., Lee P. R., Tachibana Y., Ono R., Sugio S., Tsuji Y., Tanaka Y. H., Tanaka Y. R., Masamizu Y., Hira R., Moorhouse A. J., Tamamaki N., Ikenaka K., Matsukawa N., Fields R. D., Nabekura J., Matsuzaki M. (2020). Motor learning requires myelination to reduce asynchrony and spontaneity in neural activity. Glia, 68(1), 193–210. 10.1002/glia.23713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Chong S. Y., Tuck S. J., Corey J. M., Chan J. R. (2013). A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nature Protocols, 8(4), 771–782. 10.1038/nprot.2013.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Leach M. K., Redmond S. A., Chong S. Y., Mellon S. H., Tuck S. J., Feng Z. Q., Corey J. M., Chan J. R. (2012). A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nature Methods, 9(9), 917–922. 10.1038/nmeth.2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavarao C. N., Moffett J. R., Moore R. A., Viola R. E., Namboodiri M. A. A., Jacobowitz D. M. (2004). Immunohistochemical localization of aspartoacylase in the rat central nervous system. Journal of Comparative Neurology, 472(3), 318–329. 10.1002/cne.20080 [DOI] [PubMed] [Google Scholar]
- Makinodan M., Rosen K. M., Ito S., Corfas G. (2012). A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science (New York, N.Y.), 337(6100), 1357–1360. 10.1126/science.1220845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandolesi G., Bullitta S., Fresegna D., De Vito F., Rizzo F. R., Musella A., Guadalupi L., Vanni V., Stampanoni Bassi M., Buttari F., Viscomi M. T., Centonze D., Gentile A. (2019). Voluntary running wheel attenuates motor deterioration and brain damage in cuprizone-induced demyelination. Neurobiology of Disease, 129, 102–117. 10.1016/j.nbd.2019.05.010 [DOI] [PubMed] [Google Scholar]
- Marins T., Rodrigues E. C., Bortolini T., Melo B., Moll J., Tovar-Moll F. (2019). Structural and functional connectivity changes in response to short-term neurofeedback training with motor imagery. Neuroimage, 194, 283–290. 10.1016/j.neuroimage.2019.03.027 [DOI] [PubMed] [Google Scholar]
- McKenzie I. A., Ohayon D., Li H., de Faria J. P., Emery B., Tohyama K., Richardson W. D. (2014). Motor skill learning requires active central myelination. Science (New York, N.Y.), 346(6207), 318–322. 10.1126/science.1254960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella D. M., Goodenough D. A., Sirkowski E., Scherer S. S., Paul D. L. (2003). Connexins are critical for normal myelination in the central nervous system. Journal of Neuroscience, 23(13), 5963–5973. 10.1523/JNEUROSCI.23-13-05963.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva K. D., Wolman D., Mensh B. D., Pax E., Buchanan J., Smith S. J., Bock D. D. (2016). A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. Elife, 5. 10.7554/eLife.15784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M. (2018). Myelin plasticity and nervous system function. Annual Review of Neuroscience, 41(1), 61–76. 10.1146/annurev-neuro-080317-061853 [DOI] [PubMed] [Google Scholar]
- Mount C. W., Monje M. (2017). Wrapped to adapt: experience-dependent myelination. Neuron, 95(4), 743–756. 10.1016/j.neuron.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely S. A., Williamson J. M., Klingseisen A., Zoupi L., Early J. J., Williams A., Lyons D. A. (2022). New oligodendrocytes exhibit more abundant and accurate myelin regeneration than those that survive demyelination. Nature Neuroscience, 25(4), 415–420. 10.1038/s41593-021-01009-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Lin X. H., Giese N., Heldin C. H., Stallcup W. B. (1996). Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. Journal of Neuroscience Research, 43(3), 299–314. [DOI] [PubMed] [Google Scholar]
- Olivares R., Montiel J., Aboitiz F. (2001). Species differences and similarities in the fine structure of the mammalian corpus callosum. Brain Behavior and Evolution, 57(2), 98–105. 10.1159/000047229 [DOI] [PubMed] [Google Scholar]
- Pan S., Mayoral S. R., Choi H. S., Chan J. R., Kheirbek M. A. (2020). Preservation of a remote fear memory requires new myelin formation. Nature Neuroscience, 23(4), 487–499. 10.1038/s41593-019-0582-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels P. J., Vanderver A., Bernard G., Wolf N. I., Dreha-Kulczewksi S. F., Deoni S. C., Bertini E., Kohlschutter A., Richardson W., Ffrench-Constant C., Kohler W., Rowitch D., Barkovich A. J. (2014). Hypomyelinating leukodystrophies: Translational research progress and prospects. Annals of Neurology, 76(1), 5–19. 10.1002/ana.24194 [DOI] [PubMed] [Google Scholar]
- Pukos N., Goodus M. T., Sahinkaya F. R., McTigue D. M. (2019). Myelin status and oligodendrocyte lineage cells over time after spinal cord injury: What do we know and what still needs to be unwrapped? Glia, 67(11), 2178–2202. 10.1002/glia.23702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. (1983). A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature, 303(5916), 390–396. 10.1038/303390a0 [DOI] [PubMed] [Google Scholar]
- Ranscht B., Clapshaw P. A., Price J., Noble M., Seifert W. (1982). Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proceedings of the National Academy of Sciences of the USA, 79(8), 2709–2713. 10.1073/pnas.79.8.2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond S. A., Mei F., Eshed-Eisenbach Y., Osso L. A., Leshkowitz D., Shen Y. A., Kay J. N., Aurrand-Lions M., Lyons D. A., Peles E., Chan J. R. (2016). Somatodendritic expression of JAM2 inhibits oligodendrocyte myelination. Neuron, 91(4), 824–836. 10.1016/j.neuron.2016.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid L. B., Sale M. V., Cunnington R., Mattingley J. B., Rose S. E. (2017). Brain changes following four weeks of unimanual motor training: Evidence from fMRI-guided diffusion MRI tractography. Human Brain Mapping, 38(9), 4302–4312. 10.1002/hbm.23514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser N., Heuschmann P., Wersching H., Breitenstein C., Knecht S., Floel A. (2008). Levodopa improves procedural motor learning in chronic stroke patients. Archives of Physical Medicine and Rehabilitation, 89(9), 1633–1641. 10.1016/j.apmr.2008.02.030 [DOI] [PubMed] [Google Scholar]
- Saab A. S., Nave K. A. (2017). Myelin dynamics: Protecting and shaping neuronal functions. Current Opinion in Neurobiology, 47, 104–112. 10.1016/j.conb.2017.09.013 [DOI] [PubMed] [Google Scholar]
- Saitta K. S., Lercher L. D., Sainato D. M., Patel A., Huang Y., McAuliffe G., Dreyfus C. F. (2021). CHPG Enhances BDNF and myelination in cuprizone-treated mice through astrocytic metabotropic glutamate receptor 5. Glia, 69(8), 1950–1965. 10.1002/glia.24003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Baptista C., Khrapitchev A. A., Foxley S., Schlagheck T., Scholz J., Jbabdi S., DeLuca G. C., Miller K. L., Taylor A., Thomas N., Kleim J., Sibson N. R., Bannerman D., Johansen-Berg H. (2013). Motor skill learning induces changes in white matter microstructure and myelination. Journal of Neuroscience, 33(50), 19499–19503. 10.1523/JNEUROSCI.3048-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S. S., Braun P. E., Grinspan J., Collarini E., Wang D. Y., Kamholz J. (1994). Differential regulation of the 2’,3’-cyclic nucleotide 3’-phosphodiesterase gene during oligodendrocyte development. Neuron, 12(6), 1363–1375. 10.1016/0896-6273(94)90451-0 [DOI] [PubMed] [Google Scholar]
- Scholz J., Klein M. C., Behrens T. E., Johansen-Berg H. (2009). Training induces changes in white-matter architecture. Nature Neuroscience, 12(11), 1370–1371. 10.1038/nn.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi S. H., Kordi M. R., Rajabi H., Malm C., Shah F., Quchan A. S. K. (2020). Exercise modulates the levels of growth inhibitor genes before and after multiple sclerosis. Journal of Neuroimmunology, 341, 577172. 10.1016/j.jneuroim.2020.577172 [DOI] [PubMed] [Google Scholar]
- Siegenthaler M. M., Berchtold N. C., Cotman C. W., Keirstead H. S. (2008). Voluntary running attenuates age-related deficits following SCI. Experimental Neurology, 210(1), 207–216. 10.1016/j.expneurol.2007.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solly S. K., Thomas J. L., Monge M., Demerens C., Lubetzki C., Gardinier M. V., Matthieu J. M., Zalc B. (1996). Myelin/oligodendrocyte glycoprotein (MOG) expression is associated with myelin deposition. Glia, 18(1), 39–48. [DOI] [PubMed] [Google Scholar]
- Steadman P. E., Xia F., Ahmed M., Mocle A. J., Penning A. R. A., Geraghty A. C., Steenland H. W., Monje M., Josselyn S. A., Frankland P. W. (2020). Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron, 105(1), 150–164 e156. 10.1016/j.neuron.2019.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B., Porta S., Haak L. L., Gallo V., Fields R. D. (2002). Adenosine: A neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron, 36(5), 855–868. 10.1016/S0896-6273(02)01067-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.-M., Beesley J., Grinspan J., Seth P., Kamholz J., Cambi F. (2000). Cell cycle arrest induced by ectopic expression of p27 is not sufficient to promote oligodendrocyte differentiation. Journal of Cellular Biochemistry, 76(2), 270–279. [DOI] [PubMed] [Google Scholar]
- Tikoo R., Osterhout D. J., Casaccia-Bonnefil P., Seth P., Koff A., Chao M. V. (1998). Ectopic expression of p27Kip1 in oligodendrocyte progenitor cells results in cell-cycle growth arrest. Journal of Neurobiology, 36(3), 431–440. [DOI] [PubMed] [Google Scholar]
- Tomassy G. S., Fossati V. (2014). How big is the myelinating orchestra? Cellular diversity within the oligodendrocyte lineage: Facts and hypotheses. Frontiers in Cellular Neuroscience, 8, 201. 10.3389/fncel.2014.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson L., Leiton C. V., Colognato H. (2016). Behavioral experiences as drivers of oligodendrocyte lineage dynamics and myelin plasticity. Neuropharmacology, 110, 548–562. 10.1016/j.neuropharm.2015.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tilborg E., de Theije C. G. M., van Hal M., Wagenaar N., de Vries L. S., Benders M. J., Rowitch D. H., Nijboer C. H. (2018). Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia, 66(2), 221–238. 10.1002/glia.23256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H., Lee P. R., Fields R. D. (2011). Control of local protein synthesis and initial events in myelination by action potentials. Science (New York, N.Y.), 333(6049), 1647–1651. 10.1126/science.1206998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H., Ortiz F. C., Woo D. H., Lee P. R., Angulo M. C., Fields R. D. (2015). Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat Commun, 6, 7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West K. L., Kelm N. D., Carson R. P., Does M. D. (2016). A revised model for estimating g-ratio from MRI. Neuroimage, 125, 1155–1158. 10.1016/j.neuroimage.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Ohayon D., McKenzie I. A., Sinclair-Wilson A., Wright J. L., Fudge A. D., Emery B., Li H., Richardson W. D. (2016). Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nature Neuroscience, 19(9), 1210–1217. 10.1038/nn.4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M. S., Zdunek S., Bergmann O., Bernard S., Salehpour M., Alkass K., Perl S., Tisdale J., Possnert G., Brundin L., Druid H., Frisen J. (2014). Dynamics of oligodendrocyte generation and myelination in the human brain. Cell, 159(4), 766–774. 10.1016/j.cell.2014.10.011 [DOI] [PubMed] [Google Scholar]
- Yoon H., Kleven A., Paulsen A., Kleppe L., Wu J., Ying Z., Gomez-Pinilla F., Scarisbrick I. A. (2016). Interplay between exercise and dietary fat modulates myelinogenesis in the central nervous system. Biochimica et Biophysica Acta, 1862(4), 545–555. 10.1016/j.bbadis.2016.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. M., Psachoulia K., Tripathi R. B., Dunn S. J., Cossell L., Attwell D., Tohyama K., Richardson W. D. (2013). Oligodendrocyte dynamics in the healthy adult CNS: Evidence for myelin remodeling. Neuron, 77(5), 873–885. 10.1016/j.neuron.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younsi A., Zheng G., Scherer M., Riemann L., Zhang H., Tail M., Hatami M., Skutella T., Unterberg A., Zweckberger K. (2020). Treadmill training improves survival and differentiation of transplanted neural precursor cells after cervical spinal cord injury. Stem Cell Research, 45, 101812. 10.1016/j.scr.2020.101812 [DOI] [PubMed] [Google Scholar]
- Yu W. P., Collarini E. J., Pringle N. P., Richardson W. D. (1994). Embryonic expression of myelin genes: Evidence for a focal source of oligodendrocyte precursors in the ventricular zone of the neural tube. Neuron, 12(6), 1353–1362. 10.1016/0896-6273(94)90450-2 [DOI] [PubMed] [Google Scholar]
- Zheng J., Sun X., Ma C., Li B. M., Luo F. (2019). Voluntary wheel running promotes myelination in the motor cortex through Wnt signaling in mice. Molecular Brain, 12(1), 85. 10.1186/s13041-019-0506-8 [DOI] [PMC free article] [PubMed] [Google Scholar]