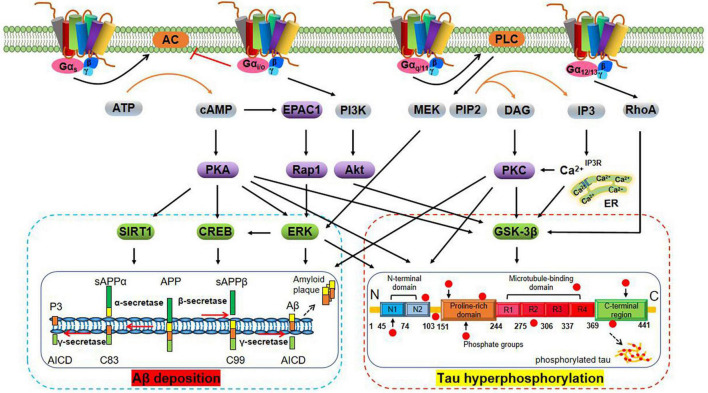
FIGURE 1.
Schematic representation of G protein regulation of Aβ deposition and tau hyperphosphorylation-related signaling pathways. Aβ is derived from the amyloidogenic cleavage of the transmembrane amyloid precursor protein (APP) mediated by α-, β-, and γ-secretases. In the amyloidogenic pathway, APP is first cleaved by the β-secretase (BACE-1), which generates soluble amyloid precursor protein β (sAPPβ) and the β-C-terminal fragment (β-CTF, also termed C99). The latter is cleaved by γ-secretase to generate the Aβ peptide and the amyloid intracellular domain (AICD). The Aβ peptide aggregates to form Aβ oligomers (oAβ) and extracellular amyloid plaques (Hamm et al., 2017). In the non-amyloidogenic pathway, cleavage of APP by α-secretases [especially A disintegrin and metalloprotease 9 (ADAM9), ADAM10, and ADAM17] generates sAPPα and carboxy-terminal fragment termed C83. Subsequent cleavage of C83 by the γ-secretase complex yields the AICD and a short fragment termed P3 (De Strooper, 2010). Tau is an axonal protein expressed in mature neurons that promotes the self-assembly of tubulin into microtubules and its stabilization. The physiological function of tau depends on its phosphorylation status and is regulated by tau protein kinase and phosphatase. In AD brains, tau hyperphosphorylation under the abnormal regulation of protein kinases [e.g., glycogen synthase kinase-3β (GSK-3β)] results in the formation of NFTs (Congdon and Sigurdsson, 2018). Activation of Gαs protein activates adenylyl cyclase (AC) and promotes cyclic adenosine monophosphate (cAMP) generation. cAMP regulates Aβ deposition and tau hyperphosphorylation via activation of the extracellular regulated protein kinase (ERK) (Angulo et al., 2003), cAMP-response element binding protein (CREB) (Wang Z. et al., 2018), silent mating type information regulation 2 homolog 1 (SIRT1), and GSK3β interaction protein/GSK3 (Ko et al., 2019; Zhang Z. et al., 2020) signaling pathways in a protein kinase A (PKA)-dependent manner (Lebel et al., 2009), and exchange protein directly activated by cAMP 1 (EPAC1)/Rap1 in a PKA-independent manner (Maillet et al., 2003). By contrast, activation of Gαi/o protein inhibits the cAMP/PKA pathway. Activation of Gβγ protein may regulate tau phosphorylation through phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB, also termed Akt)/GSK-3β pathway (Wang et al., 2016). Activation of Gαq/11 protein activates phospholipase C (PLC) to produce inositol trisphosphate (IP3) and diacylglycerol (DAG), which in turn increases concentrations of intracellular calcium (Ca2+) and activates PKC, and leads to blocking of tau hyperphosphorylation and inactivation of GSK-3β (Medeiros et al., 2011; Garwain et al., 2020). Furthermore, the Gαq/11/PLC pathway can regulate Aβ generation through the MEK/ERK/CREB pathway, among others (Wang Z. et al., 2018). Activation of Gα12/13 protein activates GSK-3β in a manner dependent on Ras homolog gene family, member A (RhoA) (Sayas et al., 2002).