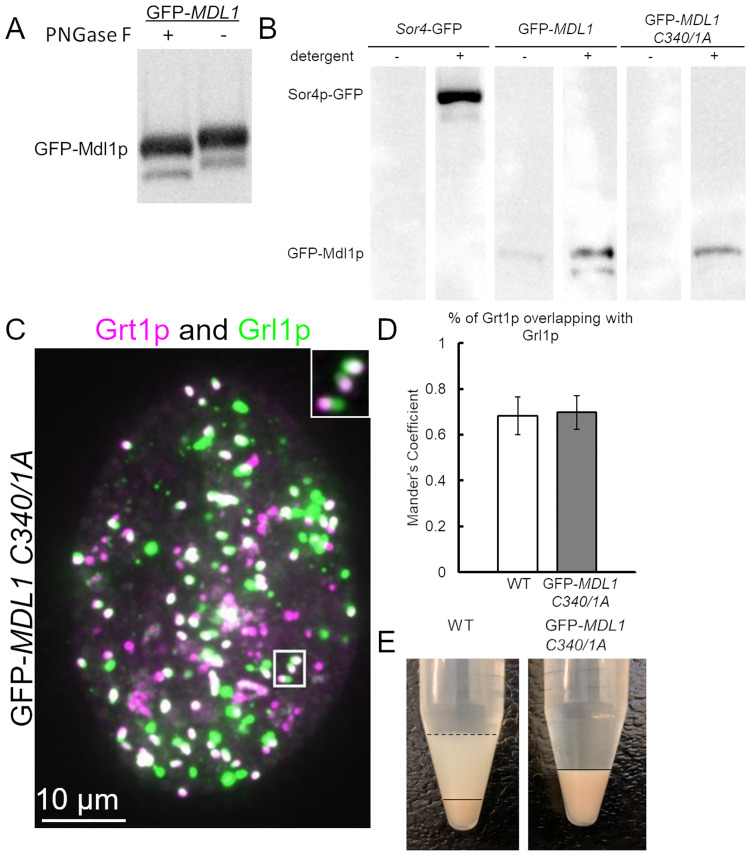
Fig 5. Membrane association of Mdl1p.
A. GFP-Mdl1p was immunoprecipitated from detergent-solubilized cell pellets derived from 50ml cultures. Bead-bound eGFP-Mdl1p was incubated with (+) or without (-) PNGase F, eluted, resolved by SDS-PAGE, and Western-blotted with anti-GFP Ab. The increase in mobility after treatment with PNGase F indicates that Mdp1p is a glycoprotein. B. Subcellular fractionation. 50 ml cultures of wildtype cells expressing Sor4p-GFP, GFP-Mdl1p, or GFP-mdl1p C340A C341A were concentrated and lysed using a ball bearing cell cracker, as described in Materials and Methods. The lysates were treated with detergent, after which the insoluble material was pelleted by ultracentrifugation. GFP-tagged proteins in the soluble fractions were immunoprecipitated, resolved by SDS-PAGE, and immunoblotted with anti-GFP Ab. Sor4p-GFP, a Type I transmembrane protein, was found in the soluble fraction only when detergent was present. For GFP-Mdl1p and GFP-mdl1p C340A C341A, a small fraction of the protein (~6%, for the former) was soluble even in the absence of detergent, while a much larger fraction was solubilized in the presence of detergent. GFP-mdl1p C340A C341A was present at lower levels than GFP-Mdl1p. C. Imaging of mucocyst localization and polarity in GFP-mdl1-C340A C341A cells. Cells were starved for 3h, and mucocysts in fixed, permeabilized cells were immunolabeled with antibodies against Grl1p (green) and Grt1p (fuschia). The mucocysts are predominantly non-docked and localized through the cytoplasm. As in WT cells, the mucocysts show polarized Grt1p concentration at one tip. Scale bar is indicated. D. Overlap between Grt1p and Grl1p localization, measured by Mander’s coefficient. As expected, the two mucocyst proteins show comparable overlap in wildtype and GFP-mdl1-C340A C341A cells. E. GFP-mdl1 C340A C341A cells are defective in induced mucocyst secretion. WT and mutant cells were stimulated with dibucaine. The secretion defect due to the cysteine-to-alanine substitutions is equivalent to that in the complete MDL1 knockout.