Abstract
Background
Gait and cognition decline with advancing age, and presage the onset of dementia. Yet, the relative trajectories of gait and cognitive decline in aging are poorly understood—particularly among those with the motoric cognitive risk (MCR) syndrome. This study compared changes in simple and complex gait performance and cognition, as a function of age and MCR.
Methods
We examined gait and cognitive functions of 1 095 LonGenity study participants (mean age = 75.4 ± 6.7 years) with up to 12 years of annual follow-up. Participants were of Ashkenazi Jewish descent, free of dementia, ambulatory, and had a 12.2% MCR prevalence at baseline. Gait speed was measured at usual pace walking (single-task walking, STW-speed) and walking while talking (WWT-speed). Eleven neuropsychological test scores were examined separately, and as a global cognition composite. Linear mixed-effects models adjusted for baseline sex, education, parental longevity, cognitive impairment, and global health were used to estimate changes in gait and cognition, as a function of age and MCR.
Results
STW-speed, WWT-speed, and cognitive tests performance declined in a nonlinear (accelerating) fashion with age. STW-speed declined faster than WWT-speed and cognitive test scores. People with MCR showed faster rates of decline on figure copy and phonemic fluency.
Conclusions
Gait declines at a faster rate than cognition in aging. People with MCR are susceptible to faster decline in visuospatial, executive, and language functions. This study adds important knowledge of trajectories of gait and cognitive decline in aging, and identifies MCR as a risk factor for accelerated cognitive decline.
Keywords: Cognitive dysfunction, Gait decline, MCR, Relative trajectories
Gait performance declines with advancing age (1) and accelerated gait decline in aging often precedes cognitive decline, mild cognitive impairment, and dementia (2,3). The relative trajectories of gait decline and cognitive decline in aging, however, are not well understood. A better understanding of the temporal interrelationship between gait and cognitive decline in aging is important for the development of more effective, targeted, and appropriately sequenced interventions to prevent or reduce gait and cognitive impairment in aging. It is also important to identify individuals that are at increased and reduced risk for accelerated gait and cognitive decline in order to determine which older adult populations may benefit from intervention. The current study aimed to address these distinct yet related issues by providing insights into the temporality of the trajectories of gait and cognitive decline in older adults.
The results of previous examinations of gait and cognitive decline trajectories in aging have yielded inconsistent results. Some studies have observed that slow gait speed is associated with decline in different cognitive functions (ie, visuospatial, executive, and/or memory functions (4–7)). Other studies suggest that poor cognition (ie, global cognition, executive function, verbal memory) is associated with decline in gait speed, but not vice versa (5,8–10). Only a few studies have examined the simultaneous decline in gait and cognitive functions in longitudinal studies. Some have examined gait and cognitive trajectories in association with dementia (11). Others have reported that cognitive decline (ie, global cognition, executive function, processing speed, and verbal memory) is associated with the slowing of gait speed (12,13). Further, distinct trajectory patterns in gait and cognition have also been identified in older adults (eg, combined decline in gait speed and global cognition vs stable cognition with declining gait) (14). To the best of our knowledge, however, no studies have examined the relative trajectories of gait and cognitive decline in aging. Examining the relative trajectories of gait and cognitive decline can provide insight into (a) whether decline in gait or cognitive functions is faster and/or occurs earlier over the course of aging, (b) the optimal timing for interventions to improve gait and cognitive functions in aging, and (c) the underlying causal pathways between age-related gait and cognitive decline.
Gait is most commonly assessed as someone’s usual walking pace (simple gait). Yet, more complex gait conditions such as walking while performing a cognitive task have also been widely used to expose cognitive and motor impairment earlier in aging—presumably because they involve divided attention and use more brain resources than simple gait (15). We hypothesized that the rate of decline in gait speed during complex gait (walking while reciting alternate letters of the alphabet) would be faster than the rate of decline in simple gait, and both simple and complex gait speed would decline faster than cognitive functions in aging. We further hypothesized that individuals with the motoric cognitive risk (MCR) syndrome may be at an increased risk for accelerated gait and cognitive decline because they are at increased risk for both Alzheimer’s disease and vascular dementia (16,17). The MCR syndrome is characterized by the presence of slow gait and subjective cognitive complaints in the absence of dementia and mobility disability (17), and the risk for MCR increases with age and as a function of physical inactivity and low education (18). Thus, the current study not only examined the relative trajectories of gait and cognitive decline as a function of aging, but also as a function of the MCR syndrome.
Method
Study Participants
The LonGenity study is a longitudinal study of Ashkenazi Jewish older adults that examines genetics and the biological mechanisms that protect from age-related diseases, and promote successful aging (19). LonGenity study participants are defined as either offspring of parents with exceptional longevity (having at least 1 parent lived to age 95 or older) or offspring of parents with usual survival. Participants are recruited through public records (ie, voter registration lists) and community advertising (ie, Jewish newsletters, contacts at synagogues). Individuals with a diagnosis of dementia (>8 on the Blessed Mental Status Examination and >2 on the AD8-item Informant Questionnaire) at initial screening, severe visual or hearing impairment, and siblings already enrolled in the study are excluded. For the present study, a subset of LonGenity participants who have been annually followed for up to 12 years (from 2008 to 2020) with gait assessments was included. Ethical clearance was obtained from the Committee on Clinical Investigations of the Albert Einstein College of Medicine. Written informed consent was obtained from all participants.
Gait Assessments
Gait speed (cm/s) was measured during simple gait (single-task walking, STW-speed) and complex gait or walking while talking (WWT-speed) with an 8.5-m-long computerized GAITRite walkway. During STW, participants completed 1 straight walk (no turning included) at their usual walking pace (20), after a practice trail. The walk started 1.2 m before and ended 1.2 m after the walkway to allow for steady pace walking. During WWT, participants were asked to complete 1 walk while reciting alternate letters of the alphabet starting with the letter “A” or “B,” depending on the month of recruitment. No practice trials were allowed and STW tests were performed first for each participant. During DTW participants were instructed to pay equal attention to walking and reciting alternate letters of the alphabet. Correct and incorrect responses while reciting alternate letters were also recorded, and the corrected response rate (CRR, correct responses per second × percentage of the correct responses) was calculated to assess cognitive performance during WWT (21). Participants wore comfortable footwear and assessments were completed in a quiet and well-lit hallway.
Cognitive Assessments
Global cognition was assessed with a standardized composite score generated from 11 different neuropsychological test scores. The individual test scores included in this composite score were separated into the following cognitive domains based on a priori knowledge (22): (a) executive function: Trail Making Test B-A (TMT interference) (23); (b) working memory: Digit Span test of the Wechsler Adult Intelligence Scale-III (WAIS-III) (24); (c) processing speed: Trail Making Test A and Digit Symbol Substitution test of WAIS-III; (d) language: Boston Naming (15-item) (25), Category and Phonemic fluency tests (26); (e) visuospatial function: Figure copy test of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (27); (f) visual memory: Figure recall test; and (g) verbal episodic memory: Free Recall on Free and Cued Selective Reminding Test (28) and Logical Memory subtest of Wechsler Memory Scale-Revised (29).
MCR Diagnosis
Motoric cognitive risk is a predementia syndrome defined as the presence of subjective cognitive complaints and slow gait speed in older adults without dementia and mobility disability (inability or required assistance with ambulation) (17). Motoric cognitive risk diagnosis was built on previously published MCR criteria (17,30). Cognitive complaints were assessed with an item of memory complaint on the 15-item Geriatric Depression Scale and a standard self-health assessment questionnaire (and verified by an informant or a clinician). We have previously published criteria for slow gait speed for the LonGenity participants, defined as walking speed 1 SD below age- and sex-specific means (17). Slow gait speed was defined as <101.9 cm/s for men and <97.4 cm/s for women under 75 years, and <85.3 cm/s for men and <76.7 cm/s for women age ≥75 years. Motoric cognitive risk diagnoses were assigned algorithmically as described previously in the LonGenity cohort and blinded to WWT and cognitive test performance (17). This study used data from baseline to determine MCR status.
Covariates
Demographic data (age, sex, and education), medical history, and parental longevity were obtained from self-report. Summed value of presence or absence of 9 medical conditions (hypertension, diabetes, chronic heart failure, arthritis, depression, stroke, chronic obstructive pulmonary disease, myocardial infarction, angina) was calculated to create a global health score. Body mass index was calculated using participants’ height and weight. Based on neuropsychological test performance, 2 clinical neuropsychologists reached consensus on a diagnosis of cognitive impairment if a participant scored ≤ 1.5 SD of the age appropriate means (31).
Data Analysis
Stata (StataCorp LLC, College Station, TX) version 16.1 was used in all the analyses. Longitudinal associations of STW-speed, WWT-speed, and cognitive functions with age were examined using linear mixed-effects models. Age was centered using the sample mean as the centering point (to make the intercept of the models more meaningful) and was included as the predictor variable. The following outcome variables were assessed at each time point, and standardized into a z-score using the population means and standard deviations across the follow-ups: (a) STW-speed, (b) WWT-speed, (c) test scores of different neuropsychological tests, and (d) the composite measure of global cognitive function. All outcome measures were standardized as our aim was to compare the trajectories of outcome measures, that originally had different measures of units, as a function of aging. Models were adjusted for sex, education, parental longevity, baseline cognitive impairment, and history of medical conditions. We chose these demographic and clinical factors as potential confounders based on previous observations that they are associated with poorer gait (1,32), cognitive performance (33,34), and changes in brain structure (35). A quadratic term for centered age was added to the models to determine if gait and cognitive measures declined in a nonlinear fashion. Likelihood ratio tests were used to confirm the goodness of fit of models with and without the quadratic term. An interaction term of “centered age × MCR” was added in separate models to determine if the trajectories of gait speed and cognitive decline were modified by the presence of MCR at baseline. p Values of <.05 was considered significant, and false discovery rate (FDR) correction was used as an adjustment for multiple comparisons. Although our main aim was to examine the effect of overall MCR syndrome at baseline on the trajectories of gait and cognitive decline, as a sensitivity analysis we examined the effects of baseline MCR, additionally adjusting for baseline gait speed.
We also performed 2 secondary analyses. First, we examined whether the trajectories of gait and cognitive decline were markedly different in participants with few waves (≤2 waves) compared to participants with many waves (≥6 waves), using separate linear mixed-effect models. Second, we examined age-related changes in the CRR during WWT, in an additional linear mixed-effect model.
Results
One participant with possible dementia at baseline (n = 1) was excluded, leaving a final eligible sample of 1 095 of older adults. The mean number of waves was 3.9 ± 2.5 years (range 1–12 waves). Table 1 summarizes the characteristics of study participants at baseline. The mean age of the sample was 75.4 ± 6.7 years, 56.1% (n = 614) were female, and 12.2% met MCR criteria. Supplementary Table 1 summarizes the sample size and mean age of participants at each wave.
Table 1.
Characteristics of Participants at Baseline
| Whole Sample (n= 1 095) | Those Without MCR (n= 699) | Those With MCR (n=97) | ||||
|---|---|---|---|---|---|---|
| Age (years), mean, SD | 75.4 | 6.7 | 75.1 | 6.5 | 75.2 | 6.7 |
| Female, n, % | 614 | 56.1 | 406 | 58.1 | 58 | 59.8 |
| Education(years), mean, SD | 17.5 | 2.8 | 17.6 | 2.7 | 17.6 | 3.3 |
| MCR, n, % | 97 | 12.2 | ||||
| OPEL n, % | 582 | 53.3 | 384 | 54.9 | 50 | 51.6 |
| Medical conditions | ||||||
| Hypertension, n, % | 452 | 41.3 | 271 | 38.8 | 56 | 57.7 |
| Diabetes, n, % | 92 | 8.4 | 50 | 7.2 | 14 | 14.4 |
| Cardiac arrhythmias, n, % | 10 | 0.9 | 4 | 0.6 | 1 | 1.0 |
| Stroke, n, % | 38 | 3.5 | 18 | 2.6 | 6 | 6.2 |
| Parkinson’s disease, n, % | 13 | 1.2 | 6 | 0.9 | 2 | 2.1 |
| BMI, mean, SD | 26.5 | 4.7 | 26.2 | 4.5 | 28.6 | 5.7 |
| Gait measures | ||||||
| STW-speed (cm/s), mean, SD | 110.2 | 20.1 | 113.9 | 18.0 | 83.5 | 12.1 |
| WWT-speed (cm/s), mean, SD | 76.6 | 26.8 | 78.9 | 27.1 | 58.7 | 16.8 |
| Cognitive tests | ||||||
| Global cognition, mean, SD (–24.1, 13.8) | .5 | 5.8 | .9 | 5.5 | .6 | 5.0 |
| TMT B-A (seconds), mean, SD (–13, 305) | 48.2 | 35.9 | 47.1 | 33.6 | 48.0 | 36.9 |
| TMT-A (seconds), mean, SD (15–117) | 42.9 | 17.0 | 41.3 | 14.9 | 43.7 | 14.8 |
| Digit Span, mean, SD (8–30) | 17.6 | 3.7 | 17.7 | 3.7 | 17.4 | 3.7 |
| Digit Symbol, mean, SD (17–103) | 59.6 | 14.4 | 60.7 | 14.1 | 58.4 | 12.3 |
| Boston Naming, mean, SD (0–15) | 13.3 | 2.2 | 13.3 | 1.9 | 13.7 | 1.5 |
| Logical Memory, mean, SD (4–46) | 23.2 | 6.3 | 23.3 | 6.1 | 24.5 | 6.1 |
| Figure copy, mean, SD (9–20) | 18.8 | 1.7 | 18.8 | 1.6 | 18.8 | 1.67 |
| Figure recall, mean, SD (0–20) | 12.9 | 3.9 | 13.1 | 3.8 | 13.1 | 3.7 |
| Free recall, mean, SD (2–46) | 33.3 | 5.4 | 33.7 | 5.2 | 33.7 | 4.7 |
| Category fluency, mean, SD (13–88) | 46.9 | 10.8 | 47.7 | 10.7 | 47.4 | 9.4 |
| Phonemic fluency, mean, SD (11–88) | 48.0 | 12.6 | 48.7 | 12.1 | 45.8 | 13.2 |
Notes: BMI = body mass index; MCR = motoric cognitive risk syndrome; OPEL = offspring of parents with exceptional longevity; SD = standard deviation; STW-speed = gait speed during single-task walking; TMT = Trial Making Test; WWT-speed = gait speed during walking while talking. The LonGenity study was composed of highly educated older adults (majority female) with low disease comorbidity, except for hypertension. The MCR prevalence was 12.2%. Participants had good gait speed and neuropsychological test scores at baseline. Compared to those without MCR at baseline, those with MCR had greater BMI, and slower walking speeds (both STW, WWT), but there was no difference in neuropsychological test scores.
The Relative Trajectories of Gait and Cognitive Decline With Age
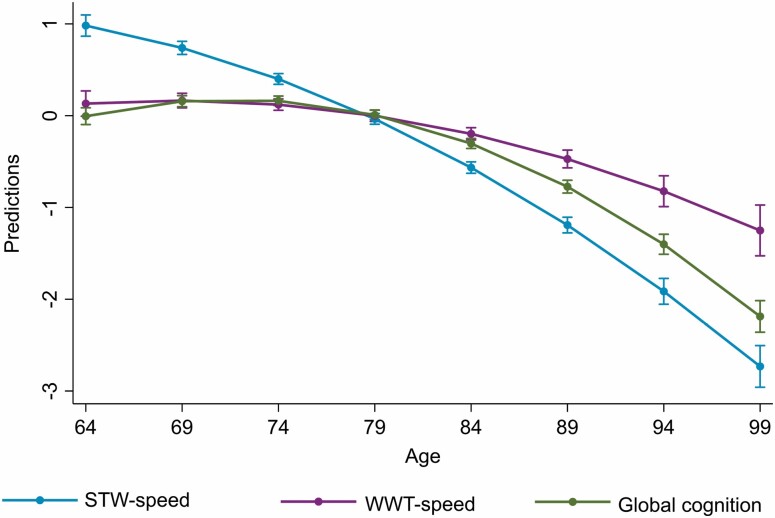
An overall (accelerating) nonlinear decline with age was observed in STW-speed, WWT-speed, and all cognitive functions, after adjusting for sex, level of education, parental longevity, baseline cognitive impairment, and history of medical conditions (Figure 1). Table 2 summarizes the rates of change in the standardized measures of STW-speed, WWT-speed, and cognitive functions. Because the trajectories of gait and cognitive decline were found to be nonlinear, based on visual inspection and statistical testing with a likelihood ratio test, we provide the effect of age on gait and cognition at 68.3, 73.4, 78, 83.2, 89.8 years, corresponding to the 5th, 25th, 50th, 75th and 95th percentiles of the age variable within our cohort (Table 2). We found that STW-speed decline occurs even during earlier ages, and declines at a faster rate than WWT-speed and performance on all cognitive tasks (Table 2; Figure 1).
Figure 1.
Decline in gait speed during single-task walking (STW-speed) and walking while talking (WWT-speed) and global cognition with aging (Note that in order to compare the trajectories between gait and cognitive decline the predictions are based on standardized measures of gait speeds and global cognition. Variables were standardized using the overall sample mean).
Table 2.
Longitudinal Associations Between Age and Standardized Performance in Gait and Cognitive Tests at Age Percentiles 5, 25, 50, 75, and 95
| β (95% CI) at 68.3 Years (5th percentile) | p | β (95% CI) at 73.4 Years (25th percentile) | p | β (95% CI) at 78 Years (50th percentile) | p | β (95% CI) at 83.2 Years (75th percentile) | p | β (95% CI) at 89.8 Years (95th percentile) | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| STW-speed | −0.055 (−0.066, −0.044) | <.001 | −0.074 (−0.081, −0.067) | <.001 | −0.091 (−0.096, −0.086) | <.001 | −0.110 (−0.117, −0.104) | <.001 | −0.135 (−0.148, −0.123) | <.001 |
| WWT-speed | 0.002 (−0.012, 0.015) | .815 | −0.014 (−0.022, −0.005) | .001 | −0.027 (−0.033, −0.022) | <.001 | −0.043 (−0.051, −0.035) | <.001 | −0.063 (−0.078, −0.047) | <.001 |
| Global cognition | 0.021 (0.013, 0.030) | <.001 | −0.011 (−0.016, −0.006) | <.001 | −0.040 (−0.043, −0.036) | <.001 | −0.073 (−0.078, −0.067) | <.001 | −0.114 (−0.124, −0.105) | <.001 |
| TMT interference | −0.002 (−0.016, 0.011) | .735 | 0.021 (0.012, 0.029) | <.001 | 0.042 (0.036, 0.047) | <.001 | 0.065 (0.057, 0.073) | <.001 | 0.095 (0.080, 0.111) | <.001 |
| TMT-A | −0.014 (−0.026, −0.003) | .013 | 0.012 (0.005, 0.019) | .001 | 0.036 (0.031, 0.041) | <.001 | 0.062 (0.056, 0.069) | <.001 | 0.097 (0.084, 0.109) | <.001 |
| Digit Span | 0.009 (−0.001, 0.019) | .082 | −0.002 (−0.009, 0.004) | .444 | −0.013 (−0.017, −0.008) | <.001 | −0.025 (−0.031, −0.018) | <.001 | −0.040 (−0.051, −0.028) | <.001 |
| Digit Symbol | −0.014 (−0.022, 0.005) | .002 | −0.034 (−0.039, −0.028) | <.001 | −0.052 (−0.056, −0.048) | <.001 | −0.072 (−0.078, −0.067) | <.001 | −0.099 (−0.108, −0.089) | <.001 |
| Boston Naming | 0.018 (0.005, 0.031) | .006 | −0.005 (−0.013, 0.003) | .261 | −0.025 (−0.031, −0.020) | <.001 | −0.049 (−0.056, −0.041) | <.001 | −0.078 (−0.092, −0.064) | <.001 |
| Logical Memory | 0.022 (0.010, 0.033) | <.001 | −0.001 (−0.008, 0.006) | .785 | −0.022 (−0.026, −0.017) | <.001 | −0.045 (−0.052, −0.038) | <.001 | −0.074 (−0.087, −0.061) | <.001 |
| Figure copy | 0.0001 (−0.014, 0.014) | .991 | −0.016 (−0.024, −0.007) | .001 | −0.030 (−0.036, −0.025) | <.001 | −0.046 (−0.054, −0.038) | <.001 | −0.067 (−0.082, −0.051) | <.001 |
| Figure recall | 0.001 (−0.011, 0.014) | .817 | −0.018 (−0.025, −0.010) | <.001 | −0.035 (−0.040, −0.030) | <.001 | −0.055 (−0.062, −0.047) | <.001 | −0.079 (−0.093, −0.066) | <.001 |
| Free recall | 0.016 (0.005, 0.028) | .006 | −0.004 (−0.012, 0.003) | .258 | −0.023 (−0.028, −0.018) | <.001 | −0.044 (−0.051, −0.037) | <.001 | −0.071 (−0.084, −0.058) | <.001 |
| Category fluency | −0.003 (−0.013, 0.008) | .593 | −0.019 (−0.026, −0.013) | <.001 | −0.034 (−0.039, −0.030) | <.001 | −0.051 (−0.057, −0.044) | <.001 | −0.072 (−0.084, −0.060) | <.001 |
| Phoneme fluency | 0.026 (0.016, 0.036) | <.001 | 0.014 (0.008, 0.020) | <.001 | 0.004 (−0.001, 0.008) | .116 | −0.009 (−0.015, −0.003) | .006 | −0.024 (−0.035, −0.013) | <.001 |
Notes: All models were adjusted for sex, level of education, parental longevity, and cognitive impairment. CI = confidence interval; STW-speed = gait speed during single-task walking; TMT = Trial Making Test; WWT-speed = gait speed during walking while talking. Higher scores in TMT interference and TMT-A and lower scores in other cognitive tests indicate poorer function. The number of participants in each age percentile group were: age ≤ 68.3: n = 176; 68.3 > age ≤ 73.4: n = 321; 73.4 > age ≤ 78: n = 232; 78 > age ≤ 83.2: n = 205; 83.2 > age ≤ 89.8: n = 133. Gait speed during STW and WWT, global cognition, and test scores of individual neuropsychological tests show a nonlinear decline with aging. Decline in STW-speed occurs earlier in aging (even during late 60s), whereas decline in WWT-speed and cognitive tests occur around the age 73.
Effect Modification by MCR at Baseline
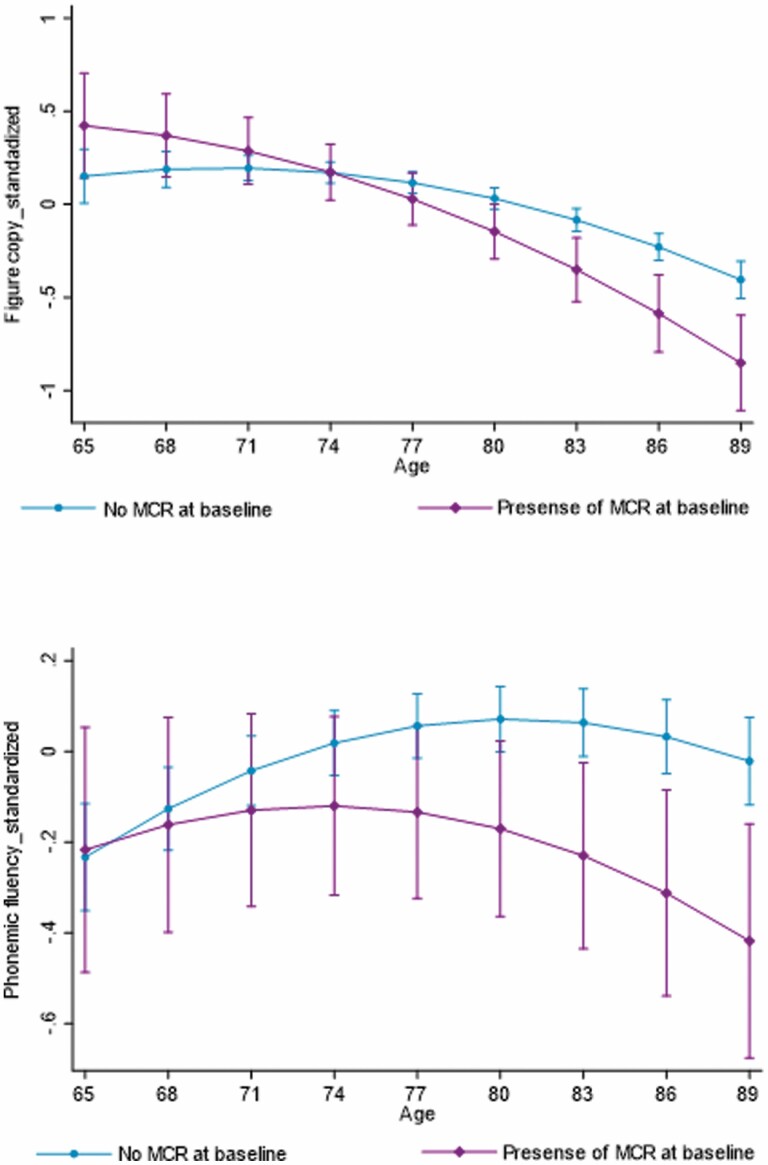
Meeting MCR criteria at baseline was associated with faster rate of decline in figure copy (β = –0.026, 95% confidence interval [CI]: –0.045, –0.007, p = .006) and phonemic fluency test performance (β = –0.017, 95% CI: –0.033, –0.002, p = .029) and a slower rate of decline in STW-speed (β = 0.038, 95% CI: 0.022, 0.054, p < .001) and WWT-speed (β = 0.022 95% CI: 0.002, 0.041, p = .031; Table 3 and Figure 2). Only the effect modifications on figure copy and STW-speed remained significant after FDR correction for multiple comparisons. Supplementary Table 2 summarizes the effects of MCR syndrome on the trajectories of gait and cognitive decline, additionally adjusted for baseline gait speed. Adjusting for baseline gait speed did not result in meaningfully different results.
Table 3.
Effect Modification on Decline in Gait and Cognitive Functions by Presence of Motoric Cognitive Risk (MCR) Syndrome
| b | 95% CI | p Value | FDR Critical Value | |
|---|---|---|---|---|
| STW-speed | 0.038 | 0.022, 0.054 | .000 | 0.003 |
| WWT-speed | 0.022 | 0.002, 0.041 | .031 | 0.013 |
| Global cognition | –0.009 | –0.022, 0.004 | .168 | 0.023 |
| TMT interference | –0.011 | –0.030, 0.009 | .273 | 0.027 |
| TMT-A | 0.002 | –0.014, 0.018 | .838 | 0.043 |
| Digit Span | –0.012 | –0.028, 0.004 | .144 | 0.020 |
| Digit Symbol | –0.002 | –0.016, 0.011 | .718 | 0.037 |
| Boston Naming | 0.001 | –0.017, 0.019 | .916 | 0.047 |
| Logical Memory | –0.003 | –0.020, 0.014 | .752 | 0.040 |
| Figure copy | –0.026 | –0.045, –0.007 | .006 | 0.007 |
| Figure recall | 0.003 | –0.015, 0.022 | .712 | 0.033 |
| Free recall | –0.015 | –0.033, 0.002 | .091 | 0.017 |
| Category fluency | –0.009 | –0.025, 0.007 | .282 | 0.030 |
| Phonemic fluency | –0.017 | –0.033, –0.002 | .029 | 0.010 |
Notes: CI = confidence interval; FDR = false discovery rate correction: a corrected p value that is adjusted for multiple comparisons. The associations with p values less than the FDR-corrected p values are considered to survive the adjustments for multiple comparisons. STW-speed = gait speed during single-task walking; TMT, Trial Making Test; WWT-speed = gait speed during walking while talking. Higher scores in TMT interference and TMT-A indicate poorer function.
Figure 2.
Decline in figure copy and phonemic fluency test performance in those with and without the motoric cognitive risk (MCR) syndrome at baseline.
The Relative Trajectories of Gait and Cognitive Decline in Those With Few and Many Waves
The trend in relative trajectories followed a similar pattern (eg, decline in STW-speed > global cognition > WWT-speed) in participants with few waves (≤2) and in those with many waves (≥6; see Supplementary Table 3). Only linear trajectories could be examined in those with few (only 2) waves.
Decline in Cognitive Performance During WWT
In a subsample of participants with data on cognitive performance during WWT (n = 743), the corrected response rate declined in a nonlinear fashion (Supplementary Figure 1). Decline corresponding to the age percentiles were as follows: at 68.3 years β = –0.031, 95% CI: –0.048, –0.014, p < .001; at 73.4 years β = –0.013, 95% CI: –0.023, –0.003, p = .014; at 78 years β = –0.003, 95% CI: –0.003, 0.010, p = .336; at 83.2 years β = 0.022, 95% CI: 0.012, 0.032, p < .001; at 89.8 years β = 0.044, 95% CI: –0.026, 0.063, p < .001.
Discussion
The results of this study suggest that trajectories of gait and cognitive decline in aging are nonlinear, and that single-task walking speed (STW-speed) declines faster than gait speed during walking while talking (WWT-speed) and a number of different cognitive functions—including global cognition. STW-speed starts to decline during “early” aging (<73 years), whereas WWT-speed and cognitive functions start to decline during “later” aging (>73). For all measures studied here, decline accelerated during later aging. Individuals with the MCR syndrome declined faster in visuospatial, executive, and language functions, but had a slower rate of decline in gait speed. The implications of these findings are discussed in more detail next.
Gait and Cognitive Decline as a Function of Age
As hypothesized, decline in STW-speed was faster than decline in all cognitive functions and occurred earlier in aging. This is in line with that slow gait speed at baseline (4,6,7) or early years of follow-up (36,37) predicted decline in global cognition (13,38) and specific cognitive domains (ie, executive function (4,5), processing speed (6,7), and visuospatial function (4,6,7)). In the literature, associations between gait speed and memory decline are inconsistent, however. Some studies have reported that slow gait speed was associated with greater memory decline (5); whereas others have reported no association (4) or associations seen only in those with ApoE4 allele (6). The inconsistency in the results may be explained by the differences in sample sizes (4–6), inclusion of only women (5), and the neuropsychological tests that have been used to assess memory (ie, Hopkins Verbal Learning test (5,6) vs Wechsler Memory Scale-Revised (4)). Our results, from a large sample (n = 1 095) of initially cognitive-healthy older adults followed for up to 12 years, provide novel information that STW-speed decline occurs earlier in aging, and declines at a faster rate compared to global cognition and a number of different cognitive functions, including memory. This study also confirms prior findings that slow gait speed can be a predictor of cognitive decline in specific domains. In a recent study of Mexican and European American older adults, however, a majority (65.4%) showed stable performance in usual pace gait speed and global cognition assessed with Mini-Mental State Examination (14). Although this prior study had a longer follow-up (mean 9.5 years), the differences in findings may be explained by the younger age span (age 65–74 years vs 62–94 in the present study), smaller sample size (n = 370 vs 1 095), and differences in gait and cognitive assessments.
In contrast to what was hypothesized STW-speed declined faster than WWT-speed in this study. In some prior studies, slow WWT-speed, but not STW-speed, was associated with poorer cognition, (39) and increased risk of dementia (40)—suggesting that WWT may be more cognitively demanding. Currently, there is limited understanding as to how WWT-speed declines with advancing age among cognitively-healthy older adults. Our study is one of the few that reported WWT-speed declined over time and additionally that the rate of decline was slower compared to STW-speed. One explanation for the differences in the rates of decline could be that STW-speed was initially faster than WWT-speed, therefore had a greater range to decline. It is also possible that STW-speed is affected by decline in multiple physiological systems such as muscular (ie, reduced muscle strength) and pulmonary (ie, reduced aerobic function) (41) and therefore show a faster decline. Although WWT-speed could be affected by these functional impairments, it is possible that during WWT participants may have prioritized gait—or employed a more cautious gait—in order to maintain balance and prevent falls in the presence of impaired physical function. Our results on decline in the cognitive performance during WWT lend some support to this suggestion. Hence, this finding combined with the initially slower WWT-speed with a lesser range to decline might have resulted in a slower rate in the trajectory of WWT-speed compared to STW-speed.
Gait and Cognitive Decline as a Function of MCR
Presence of MCR was associated with faster decline in figure copy and phonemic fluency performance—although phonemic fluency did not survive our FDR correction. Figure copy and phonemic fluency are tests of visuospatial function and verbal fluency (language), respectively (22). Both these tests, however, also demand executive functions such as planning and organization. For example, organizing the orientation and location of components is needed for figure copy while phonemic fluency tests someone’s ability to seek strategy for word generation (42). Additionally, phonemic fluency may also assess some aspects of verbal memory (42). Hence, these findings are consistent with the fact that MCR is a predictor of both vascular dementia and Alzheimer’s disease (primarily affecting executive function and memory, respectively) (16,30). Further, we add to that knowledge that people with MCR may have faster rate of decline in executive and visuospatial function, compared to those without (18). Although the definition of MCR is gait-based, its presence was not associated with a faster rate of decline in STW- or WWT-speed. By contrast, those with MCR showed a slower rate of decline, possibly due to that they already have slower gait speeds that leave a limited range to decline over time. This result is consistent with the prior finding that MCR is more of a cognitive syndrome with physical features, and that cognitive, not motor impairment, predicts transition from MCR to dementia (43).
Clinical and Research Implications
We provide novel insights into the current knowledge base that the decline in STW-speed occurs earlier in aging and that the trajectory of STW-speed decline is faster compared to decline in cognitive functions. Along with prior studies, this confirms the potential of gait speed as a marker of future cognitive decline. STW-speed can be easily measured at the clinic with little equipment and low cost. Additionally, clinically meaningful cut scores for slow gait speed (<1 m/s) (44) and decline in gait speed (0.05 m/s annual decline) (45), as a marker of adverse health outcomes are already identified. Interestingly, by contrast to some prior studies (4), greater decline in gait speed was observed relative to decline in both memory- and nonmemory-related cognitive functions; therefore, speed may be incorporated into risk assessment toolkits of both Alzheimer’s disease and vascular dementia. Furthermore, given that gait speed is easy, quick to assess, it might also be possible to promote population-based approaches to facilitate gait speed measurement. For example, allowing older people to measure gait speed on their own (ie, with a measured distance and a touch timer in shopping malls) and discuss at doctors’ visits might provide a wider screening opportunity to identify those at risk of cognitive decline. For those with slower gait speeds, wearable sensors may offer a potentially low-cost measure to monitor gait speed decline over time.
Another important research implication of this difference in the trajectories of decline lies in the understanding of causal pathways between gait and cognitive decline. Changes in underlying brain structure are associated with poorer gait and cognitive performance. In prior studies, we found that specific gray matter covariance patterns and smaller cortical thickness in multiple brain regions (including prefrontal, supplementary motor, the precuneus, and the insula) were associated not only with slower gait speed (46,47) and poorer cognitive performance (46), but also with the MCR syndrome (combined poor gait and cognition) (48). It remains unclear whether gait decline may be an epiphenomenon of change in brain structure or whether brain structure may be associated with gait decline through decline in cognitive functions. Although these relationships remain to be explored, current findings may indicate it is unlikely that the effects of brain structure on gait decline could be mediated through impaired cognition because our findings highlight that STW-speed decline occurs earlier in aging than cognitive decline. Future research is needed to examine the relative trajectories of brain, gait, and cognitive decline in aging. Such studies will provide further insights into the causal pathways, and whether changes in the brain precede gait decline or cognitive decline.
Strengths and Limitations
Examining the relative trajectories of gait and cognitive decline, overall and as a function of MCR syndrome, is novel. In contrast to prior studies of smaller samples of older adults, followed up over shorter periods of time (8,11), this study included a larger sample of genetically homogenous older adults and a longer follow-up period. Further, we examined decline in gait speed during both simple and complex walking conditions as well as decline on a wide array of neuropsychological test scores. There are a few limitations to be noted, however. The LonGenity study sample is composed of well-educated (mean 17.5 ± 2.8 years) healthy older adults (eg, lower levels of disease and comorbidities). As a result of the LonGenity gene discovery study design, half of the sample may benefit from inheriting healthy intrinsic factors (ie, genes) from their long-lived parents. Therefore, the trajectories of decline in gait and cognition could be slower than those in the general community-dwelling older adult population. Even in this healthy longevity-enriched sample of older adults, however, we found significant gait and cognitive decline with aging, and that the decline in STW-speed occurred earlier and at a faster rate compared to cognitive decline. The MCR prevalence in this study (12.2%) was slightly higher than the overall prevalence observed in our multicohort study (9.7%), yet well within the range of MCR prevalence that was observed in the different cohorts (2%–16%) (17). A sample of people with higher MCR prevalence may have allowed us to detect effect modifications in some of the other variables as well. Finally, although we have data on cognitive task performance during WWT, we do not have data on single-task cognitive performance. Therefore, we cannot determine the percentage difference in cognitive performance between single task and WWT (cognitive cost). Based on data from cognitive performance during WWT, it is possible that the participants may have prioritized gait over cognitive performance, but this needs to be confirmed in future studies.
In the light of our findings, it would be interesting to examine in future research how different domains of gait (eg, rhythm, variability) and walking speed reserve (the difference between usual pace and fast pace walking speed) would decline relative to cognitive functions, and how both gait and cognitive decline would occur relative to decline in brain structure and function.
Conclusion
Decline in gait speed during single-task walking occurs earlier in aging, and at a faster rate compared to decline in cognitive functions. People with MCR are more susceptible to have faster rate of decline in executive function, visuospatial function, and language, but not to decline in gait speed. This study adds important knowledge to the relative trajectories of gait and cognitive decline in aging and identifies MCR as a risk factor of accelerated age-related cognitive decline.
Supplementary Material
Contributor Information
Oshadi Jayakody, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York, USA.
Monique Breslin, Menzies Institute for Medical Research, University of Tasmania, Tasmania, Australia.
Emmeline Ayers, Department of Neurology, Albert Einstein College of Medicine, Bronx, New York, USA.
Joe Verghese, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York, USA; Department of Neurology, Albert Einstein College of Medicine, Bronx, New York, USA.
Nir Barzilai, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York, USA; Department of Genetics, Albert Einstein College of Medicine, Bronx, New York, USA.
Sofiya Milman, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York, USA; Department of Genetics, Albert Einstein College of Medicine, Bronx, New York, USA.
Erica Weiss, Department of Neurology, Albert Einstein College of Medicine, Bronx, New York, USA.
Helena M Blumen, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York, USA; Department of Neurology, Albert Einstein College of Medicine, Bronx, New York, USA.
Funding
This work was supported by grants from National Institute of Health (NIH) R01AG062659-01A1, NIH/National Institute on Aging (PI: Helena M. Blumen), R01AG057548-01A1 (PI: Joe Verghese), R01AG061155-01 (PI: Sofiya Milman), and R01AG057909 (PI: Nir Barzilai).
Conflict of Interest
None declared.
Author Contributions
Author contributions included conception and study design (H.M.B.), data collection or management (E.A., E.W.), statistical analysis (O.J., H.M.B., M.B.), interpretation of results (O.J., H.M.B., J.V.), drafting the manuscript work or revising it critically for important intellectual content (all authors) and approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (all authors).
Study participants: The LonGenity Study research staff and volunteers
References
- 1. Jayakody O, Breslin M, Srikanth V, Callisaya M. Medical, sensorimotor and cognitive factors associated with gait variability: a longitudinal population-based study. Front Aging Neurosci. 2018;10:419. doi: 10.3389/fnagi.2018.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quan M, Xun P, Chen C, et al. Walking pace and the risk of cognitive decline and dementia in elderly populations: a meta-analysis of prospective cohort studies. J Gerontol A Biol Sci Med Sci. 2017;72(2):266–270. doi: 10.1093/gerona/glw121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67(8):980–986. doi: 10.1001/archneurol.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68(8):929–937. doi: 10.1093/gerona/gls256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krall JR, Carlson MC, Fried LP, Xue QL. Examining the dynamic, bidirectional associations between cognitive and physical functioning in older adults. Am J Epidemiol. 2014;180(8):838–846. doi: 10.1093/aje/kwu198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jayakody O, Breslin M, Srikanth VK, Callisaya ML. Gait characteristics and cognitive decline: a longitudinal population-based study. J Alzheimers Dis. 2019;71(s1):S5–S14. doi: 10.3233/JAD-181157 [DOI] [PubMed] [Google Scholar]
- 7. Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29(3-4):156–162. doi: 10.1159/000111577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Best JR, Davis JC, Liu-Ambrose T. Longitudinal analysis of physical performance, functional status, physical activity, and mood in relation to executive function in older adults who fall. J Am Geriatr Soc. 2015;63(6):1112–1120. doi: 10.1111/jgs.13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atkinson HH, Rapp SR, Williamson JD, et al. The relationship between cognitive function and physical performance in older women: results from the Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2010;65(3):300–306. doi: 10.1093/gerona/glp149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watson NL, Rosano C, Boudreau RM, et al. ; Health ABC Study. Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65(10):1093–1100. doi: 10.1093/gerona/glq111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montero-Odasso M, Speechley M, Muir-Hunter SW, et al. Motor and cognitive trajectories before dementia: results from gait and brain study. J Am Geriatr Soc. 2018;66(9):1676–1683. doi: 10.1111/jgs.15341 [DOI] [PubMed] [Google Scholar]
- 12. Callisaya ML, Blizzard CL, Wood AG, Thrift AG, Wardill T, Srikanth VK. Longitudinal relationships between cognitive decline and gait slowing: the Tasmanian Study of Cognition and Gait. J Gerontol A Biol Sci Med Sci. 2015;70(10):1226–1232. doi: 10.1093/gerona/glv066 [DOI] [PubMed] [Google Scholar]
- 13. Gale CR, Allerhand M, Sayer AA, Cooper C, Deary IJ. The dynamic relationship between cognitive function and walking speed: the English Longitudinal Study of Ageing. Age (Dordr). 2014;36(4):1–11. doi: 10.1007/s11357-014-9682-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonzales MM, Wang CP, Quiben M, et al. Joint trajectories of cognition and gait speed in Mexican American and European American older adults: the San Antonio longitudinal study of aging. Int J Geriatr Psychiatry. 2020;35(8):897–906. doi: 10.1002/gps.5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ayers EI, Tow AC, Holtzer R, Verghese J. Walking while talking and falls in aging. Gerontology. 2014;60(2):108–113. doi: 10.1159/000355119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68(4):412–418. doi: 10.1093/gerona/gls191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verghese J, Annweiler C, Ayers E, et al. Motoric cognitive risk syndrome multicountry prevalence and dementia risk. Neurology. 2014;83(8):718–726. doi: 10.1212/WNL.0000000000001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Doi T, Verghese J, Shimada H, et al. Motoric cognitive risk syndrome: prevalence and risk factors in Japanese seniors. J Am Med Dir Assoc. 2015;16(12):1103.e21–1103.e25. doi: 10.1016/j.jamda.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 19. Zhang WB, Aleksic S, Gao T, et al. Insulin-like growth factor-1 and IGF binding proteins predict all-cause mortality and morbidity in older adults. Cells. 2020;9(6):1368. doi: 10.3390/cells9061368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verghese J, Buschke H, Viola L, et al. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc. 2002;50(9):1572–1576. doi: 10.1046/j.1532-5415.2002.50415.x [DOI] [PubMed] [Google Scholar]
- 21. Hall CD, Echt KV, Wolf SL, Rogers WA. Cognitive and motor mechanisms underlying older adults’ ability to divide attention while walking. Phys Ther. 2011;91(7):1039–1050. doi: 10.2522/ptj.20100114 [DOI] [PubMed] [Google Scholar]
- 22. Strauss E, Sherman EM, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; 2006. [Google Scholar]
- 23. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Ski. 1958;8(3):271–276. doi: 10.2466/pms.1958.8.3.271 [DOI] [Google Scholar]
- 24. Weschler D. Wechsler Adult Intelligence Scale-III (WAIS-III). New York, NY: The Psychological Corporation, 1997. [Google Scholar]
- 25. Williams BW, Mack W, Henderson VW. Boston Naming test in Alzheimer’s disease. Neuropsychologia. 1989;27(8):1073–1079. doi: 10.1016/0028-3932(89)90186-33932(89)90186-3 [DOI] [PubMed] [Google Scholar]
- 26. Ruff RM, Light RH, Parker SB, Levin HS. Benton controlled oral word association test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–338. doi: 10.1093/arclin/11.4.329 [DOI] [PubMed] [Google Scholar]
- 27. Duff K, Beglinger LJ, Schoenberg MR, et al. Test-retest stability and practice effects of the RBANS in a community dwelling elderly sample. J Clin Exp Neuropsychol. 2005;27(5):565–575. doi: 10.1080/13803390490918363 [DOI] [PubMed] [Google Scholar]
- 28. Zimmerman ME, Katz MJ, Wang C, et al. Comparison of “word” vs.“picture” version of the free and cued selective reminding test (FCSRT) in older adults. Alzheimer’s Dement: Diagn Assess Dis Monit. 2015;1(1):94–100. doi: 10.1016/j.dadm.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elwood RW. The Wechsler memory scale-revised: psychometric characteristics and clinical application. Neuropsychol Rev. 1991;2(2):179–201. doi: 10.1007/BF01109053 [DOI] [PubMed] [Google Scholar]
- 30. Verghese J, Ayers E, Barzilai N, et al. Motoric cognitive risk syndrome: multicenter incidence study. Neurology. 2014;83(24):2278–2284. doi: 10.1212/WNL.0000000000001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63(8):916–924. doi: 10.1001/archpsyc.63.8.916 [DOI] [PubMed] [Google Scholar]
- 32. Ayers E, Barzilai N, Crandall JP, Milman S, Verghese J. Association of family history of exceptional longevity with decline in physical function in aging. J Gerontol A Biol Sci Med Sci. 2017;72(12):1649–1655. doi: 10.1093/gerona/glx053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koran MEI, Wagener M, Hohman TJ; Alzheimer’s Neuroimaging Initiative. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 2017;11(1):205–213. doi: 10.1007/s11682-016-9523-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lipton RB, Hirsch J, Katz MJ, et al. Exceptional parental longevity associated with lower risk of Alzheimer’s disease and memory decline. J Am Geriatr Soc. 2010;58(6):1043–1049. doi: 10.1111/j.1532-5415.2010.02868.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62(8):847–855. doi: 10.1016/j.biopsych.2007.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Best JR, Liu-Ambrose T, Boudreau RM, et al. ; Health, Aging and Body Composition Study. An evaluation of the longitudinal, bidirectional associations between gait speed and cognition in older women and men. J Gerontol A Biol Sci Med Sci. 2016;71(12):1616–1623. doi: 10.1093/gerona/glw066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tian Q, An Y, Resnick SM, Studenski S. The relative temporal sequence of decline in mobility and cognition among initially unimpaired older adults: results from the Baltimore Longitudinal Study of Aging. Age Ageing. 2017;46(3):445–451. doi: 10.1093/ageing/afw185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Savica R, Wennberg A, Hagen C, et al. Comparison of gait parameters for predicting cognitive decline: the Mayo clinic study of aging. J Alzheimer’s Dis. 2017;55(2):559–567. doi: 10.3233/JAD-160697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muir SW, Speechley M, Wells J, Borrie M, Gopaul K, Montero-Odasso M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: the effect of dual-task challenges across the cognitive spectrum. Gait Posture. 2012;35(1):96–100. doi: 10.1016/j.gaitpost.2011.08.014 [DOI] [PubMed] [Google Scholar]
- 40. Montero-Odasso M, Sarquis-Adamson Y, Speechley M, et al. Association of dual-task gait with incident dementia in mild cognitive impairment: results from the gait and brain study. JAMA Neurol. 2017;74(7):857–865. doi: 10.1001/jamaneurol.2017.0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosso AL, Sanders JL, Arnold AM, et al. Multisystem physiologic impairments and changes in gait speed of older adults. J Gerontol A Biol Sci Med Sci. 2015;70(3):319–324. doi: 10.1093/gerona/glu176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lezak MD, Howieson DB, Loring DW, Fischer JS. Neuropsychological Assessment. Oxford University Press; 2004. [Google Scholar]
- 43. Verghese J, Wang C, Bennett DA, Lipton RB, Katz MJ, Ayers E. Motoric cognitive risk syndrome and predictors of transition to dementia: a multicenter study. Alzheimers Dement. 2019;15(7):870–877. doi: 10.1016/j.jalz.2019.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60(10):1304–1309. doi: 10.1093/gerona/60.10.1304 [DOI] [PubMed] [Google Scholar]
- 45. Tian Q, Resnick SM, Mielke MM, et al. Association of dual decline in memory and gait speed with risk for dementia among adults older than 60 years: a multicohort individual-level meta-analysis. JAMA Netw Open. 2020;3(2):e1921636. doi: 10.1001/jamanetworkopen.2019.21636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Blumen HM, Brown LL, Habeck C, et al. Gray matter volume covariance patterns associated with gait speed in older adults: a multi-cohort MRI study. Brain Imaging Behav. 2019;13(2):446–460. doi: 10.1007/s11682-018-9871-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jayakody O, Breslin M, Beare R, Blumen HM, Srikanth VK, Callisaya ML. Regional associations of cortical thickness with gait variability-the Tasmanian study of cognition and gait. J Gerontol A Biol Sci Med Sci. 2020;75(8):1537–1544. doi: 10.1093/gerona/glaa118 [DOI] [PubMed] [Google Scholar]
- 48. Blumen HM, Allali G, Beauchet O, Lipton RB, Verghese J. A gray matter volume covariance network associated with the motoric cognitive risk syndrome: A multicohort MRI study. J Gerontol A Biol Sci Med Sci. 2019;74(6):884–889. doi: 10.1093/gerona/gly158 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.