Fig. 4. Gid12 fully and partially obstructs substrate placement in Chelator-GIDSR4 and GIDSR4 assemblies, respectively.
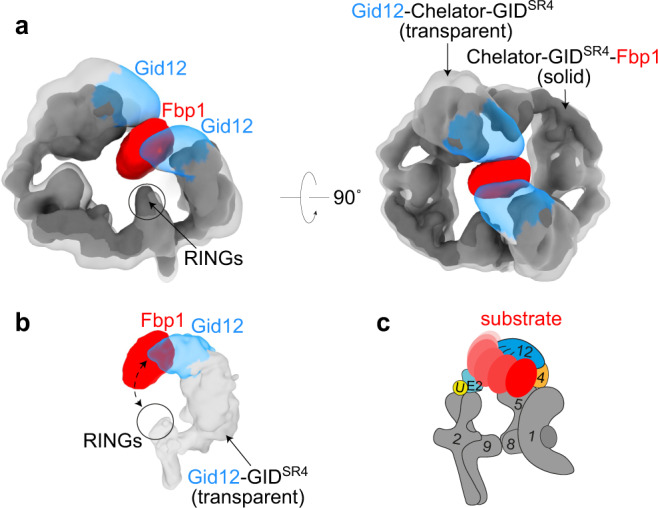
a Superposition of 3D reconstructions of Gid12-Chelator-GIDSR4 (transparent gray, with Gid12 in blue) and Chelator-GIDSR4-Fbp1 (solid gray, with substrate Fbp1 in red) shows that Gid12 fills much of the central region of Chelator-GIDSR4 that encapsulates the globular domain of the recruited Fbp1 substrate. b Superposition of 3D reconstruction of Gid12-GIDSR4 with the corresponding region of the map of Chelator-GIDSR4-Fbp1. The relatively open structure of GIDSR4 does not encapsulate Fbp1, whose globular domain may thus occupy various relative positions between the Pro/N-degron recruited to Gid4 and RING-based catalytic module. c Cartoon representation showing how GIDSR4 could dynamically accommodate various orientations of Fbp1 between Gid4 and RING-activated Ubc8~ubiquitin intermediate, some of which could be compatible with Gid12 bound to Gid4.
