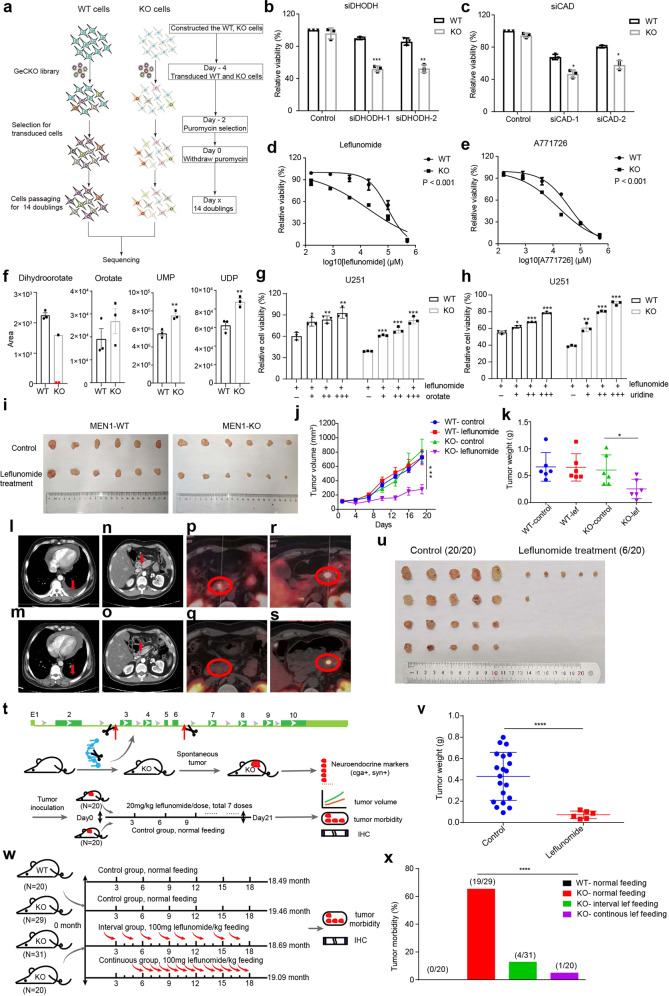
Fig. 1. Genome-wide CRISPR-Cas9 screening identifies synthetic lethal partners of MEN1 deficiency.
a Screening strategy for identification of synthetic lethal partners of MEN1 deficiency. b Relative cell viability of WT (MEN1-WT) and KO (MEN1-KO) cells with DHODH siRNA knockdown assessed with the CCK8 assay at OD 450. c Relative cell viability of WT and KO cells with CAD siRNA knockdown assessed with the CCK8 assay at OD 450. d, e Cell viability assessed with the CCK8 in WT and KO cells in the absence or presence of leflunomide or A771726 at multiple concentrations. f Relative metabolite concentrations of dihydroorotate, orotate, UMP and UDP in MEN1-WT and MEN1-KO cells. DHODH catalyzes the conversion of dihydroorotate to orotate. UMP, Uridine 5’-monophosphate; UDP, Uridine 5’-diphosphate. The red dot represents the undetectable value. g, h The relative cell viability of WT and KO cells treated with 100 µM leflunomide in combination with different concentrations of orotate (g) and uridine (h) for 48 h assessed with CCK8 assay. i Representative images of MEN1-WT and MEN1-KO U251 xenograft tumors from nude mice treated with leflunomide or vehicle control after 7 doses. j Quantification of xenograft tumor volume at different days after administration (n = 6, for both vehicle-treated and leflunomide-treated groups). k Weight of tumors harvested from animals after treatment with leflunomide or vehicle control. l Chest CT before taking the leflunomide. m Chest CT after 2 months of medication. n Abdominal CT before taking the leflunomide. o Abdominal CT after 2 months of medication. p Tumor in the head of pancreas with high SSTR2 expression (SUVmax 10.4). q Susceptive tumor in the head of pancreas with almost no SSTR2 expression (SUVmax 5.6) by GA68-PETCT at third visit. r Tumor in the tail of pancreas with high SSTR2 expression (SUVmax 27.8). s Tumor in the tail of pancreas with decreased SSTR2 expression (SUVmax 18.7). t Schematic of the construction of Men1 knockout mice with CRISPR-Cas9 technology and the subsequent drug delivery over 21 days. u Images of spontaneous tumors in Men1+/− mice under leflunomide or vehicle treatment for 7 doses. v Quantification of spontaneous tumor weight after treatment with leflunomide or control. w Schematic of the time line and leflunomide treatment for prevention of tumor development in Men1+/− mice. x Tumor morbidity for the 4 different groups of mice based on feeding with or without leflunomide: WT-normal feeding, KO-normal feeding, KO-interval treatment group and KO-continuous treatment group.