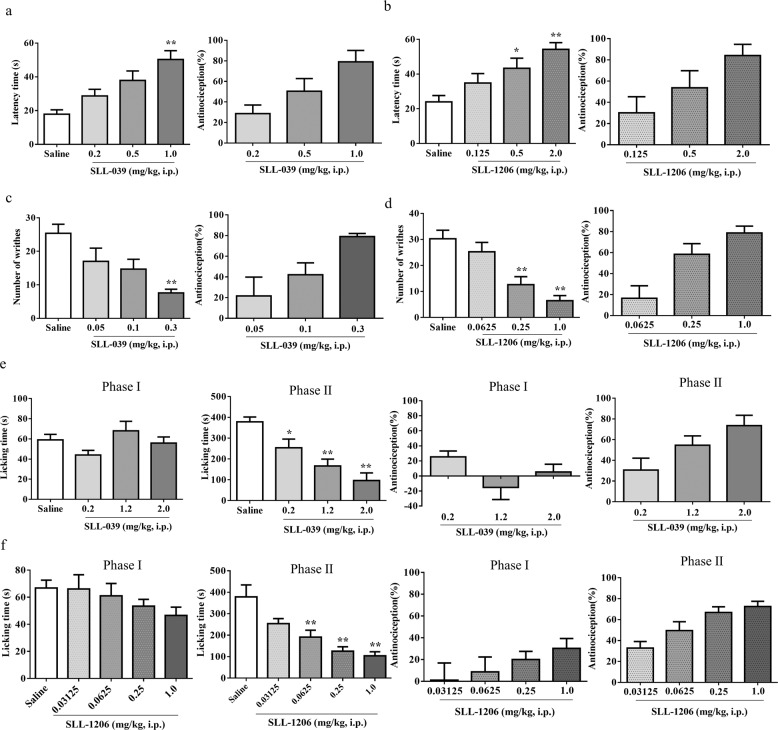
Fig. 2. The antinociceptive effects of SLL-039 and SLL-1206 were evaluated in three models.
The anti-thermal pain effects of SLL-039 and SLL-1206 were evaluated in the hot plate test. After injected with various doses of SLL-039 (a) or SLL-1206 (b), the latency time was measured 4 h later, and the antinociception (%) was calculated. SLL-039 (a) and SLL-1206 (b) dose-dependently induced anti-thermal pain effects in hot plate test. The anti-visceral pain effects of SLL-039 and SLL-1206 were evaluated in the acid writhing test. After injected with various doses of SLL-039 (−2 h) or SLL-1206 (−3 h), 0.6% acetic acid was injected (10 mL/kg body weight, i.p.). The number of writhes was counted for 20 min and the antinociception (%) was calculated. SLL-039 (c) and SLL-1206 (d) dose-dependently induced anti-visceral pain effects in acid writhing test. The anti-chemical pain and anti-inflammatory pain activities of SLL-039 and SLL-1206 were evaluated in the formalin test. After injected with various doses of SLL-039 (e) or SLL-1206 (f), 2 or 3 h later mice were injected with 20 μL 1.0% formaldehyde solution into the plantar of right hind paw and the licking time was measured for 60 min, and the antinociception (%) was calculated. SLL-039 and SLL-1206 dose-dependently induced anti-inflammatory pain in phase II. Data were analyzed by one-way ANOVA followed by Bonferoni post-hoc test. Data are presented as mean ± SEM from at least six mice. *P < 0.05, **P < 0.01 vs. saline