Figure 4.
Hrr25 regulates cell wall andmembrane stress tolerance and homeostasis through its interaction with Swi6
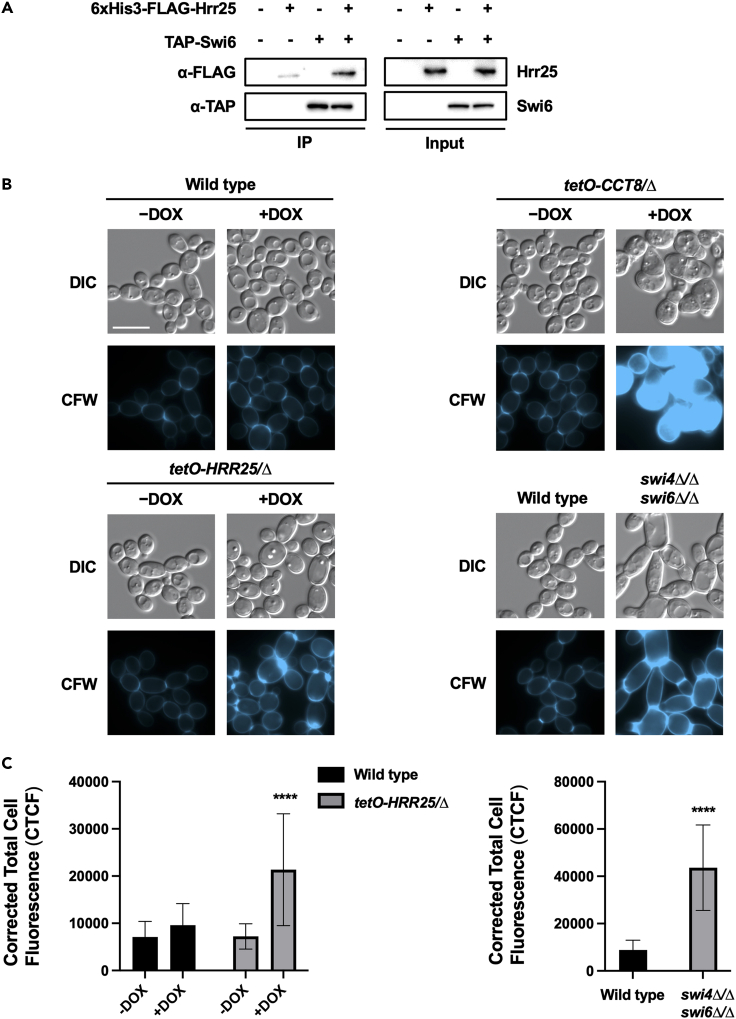
(A) Hrr25 physically associates with Swi6 in C. albicans. C-terminally TAP-tagged Swi6 was affinity purified with immunoglobulin G (IgG) beads followed by tobacco etch virus (TEV)-protease cleavage and a second round of purification using calmodulin beads. The His6-FLAG3-tagged Hrr25 was monitored by western blot analysis and detected using an α-FLAG antibody. Swi6 pull down was confirmed using an α-TAP antibody. Input samples confirm the expression of tagged proteins. Testing was repeated as an independent biological replicate to confirm results. See also Figure S3.
(B and C) Hrr25 and SBF mutants exhibit a cell wall damage response in the absence of exogenous stress. Wild type (CaSS1), tetO-HRR25/Δ, and tetO-CCT8/Δ strains were grown overnight in YPD and then subjected to an additional overnight culture in presence or absence of 0.05 μg/mL DOX. Wild type (CB420) and the swi4Δ/Δ swi6Δ/Δ strains were subject to a single overnight culture in YPD. Strains were then diluted to an OD600 of 0.1 in YPD and grown at 30°C for 4 h in shaking conditions in either the presence or absence of DOX as indicated (0.05 μg/mL for tetO-CCT8/Δ and 5 μg/mL for tetO-HRR25/Δ and wild type). Cells were stained with calcofluor white (CFW) (10 μg/mL) to visualize chitin and imaged by DIC and fluorescence microscopy. Assays were performed in biological duplicate. Scale bar indicates 10 μm and applies to all images. The Corrected Total Cell Fluorescence (CTCF) was calculated for each image using ImageJ. Means are graphed with the error bars representing SD. Signal was compared to the wild-type control and significance was determined by two-way ANOVA (Tukey’s test) or unpaired t-test. Asterisks indicate level of significance (∗∗∗∗p-value < 0.0001).