Abstract
The sperm consumes adenosine triphosphate (ATP) to maintain the cellular function, viability, acrosome reaction (AR), and motility. Extra-mitochondrial citrate synthase (eCS) catalyzes citrate production in the sperm head, and thus regulates sperm function through ATP synthesis, similarly to CS. This study aimed to investigate how eCS regulates AR. Herein, acrosome-reacted (ARed) sperms were rarely detected on the zona pellucida, and spontaneous ARed sperm in eCs -deficient (KO) sperm remained at low levels even with induced capacitation. Retarded AR of eCs -KO sperm was enhanced by cyclic adenosine 3′,5′-monophosphate (cAMP) treatment. In conclusion, eCS regulates AR via a cAMP-dependent pathway, which presumably contributes to sperm metabolism.
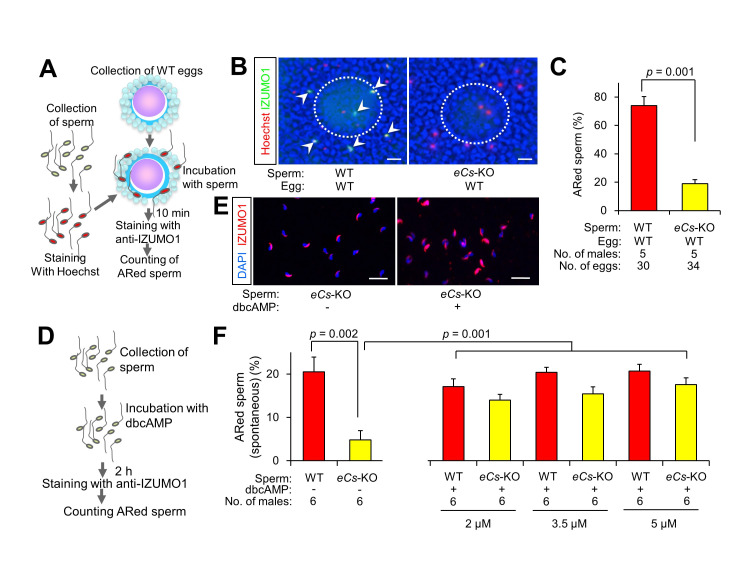
Figure 1. Retarded acrosome reaction (AR) in eCs -deficient (KO) sperm.
(A) Experimental flow. eCs -KO and wild-type (WT) sperm that were stained with Hoechst 33342 were incubated with WT eggs for 10 min and immunostained with anti-IZUMO1. (B) AR in WT and eCs -KO sperm. Acrosome-reacted (ARed) sperm were immunoreacted with Alexa Fluor 488-labeled anti-IZUMO1 monoclonal antibody and stained with Hoechst 33342. Arrowheads indicate ARed sperm. Dotted circles: WT eggs. Scale bar: 20 μm. (C) Percentage of ARed sperm. Values are expressed as mean ± SE. (D) Spontaneous ARed sperm. After WT and eCs -KO sperm were incubated without eggs in 2, 3.5, and 5 μM dibutyryl cyclic adenosine monophosphate (dbcAMP), the percentage of spontaneously ARed sperm was counted. (E) eCs -KO sperm incubated in 2 μM dbcAMP. Scale bar: 5 μm. (F) Percentage of spontaneously ARed sperm. Values are expressed as mean ± SE.
Description
Sperm must undergo sequential and complex processes in the uterus and oviducts before fertilizing the eggs, including capacitation and acrosome reaction (AR) (Gervasi and Visconti 2016). Adenosine triphosphate (ATP) production through glycolysis and oxidative phosphorylation (OXPHO) is essential for maintaining sperm motility and completing AR (Stival et al . 2016). Citrate synthase (CS) is localized in the mitochondrial matrix, where it catalyzes the reaction between acetyl coenzyme A and oxaloacetate to form citric acid (Surpin and Chory 1997). The extra-mitochondrial form of CS (eCS) is encoded by a separate gene in mice (citrate synthase like (Csl) MGI:1919082) and expressed by alternative splicing of the CS gene in humans (Kang et al . 2020). eCS also has CS activity, implying its involvement in ATP production which is related to sperm function in the extra-mitochondrial cytoplasm (Kang et al . 2020). Citrate serves as an essential substrate in the tricarboxylic acid (TCA) cycle, and its subsequent complete oxidation is the major source of cellular ATP production (Iacobazzi and Infantino 2014). Consequently, the activation of TCA cycle-related enzymes that regulate cellular ATP production may be involved in controlling sperm functions (Zhu et al . 2019). Conceivably, eCS is involved in regulating AR in the sperm head, as citrate is an important substrate in cellular energy metabolism (Iacobazzi and Infantino 2014). However, the role of eCS in AR remains unclear.
We studied the influence of eCS deficiency on AR using IZUMO1, an integral membrane protein of the sperm that is essential for sperm-egg fusion (Inoue et al . 2005). Anti-IZUMO1 monoclonal antibody (mAb) can react with the sperm head only after permeabilization because IZUMO1 is present in the inner acrosomal region before AR (Inoue et al . 2005). However, in sperm that have undergone AR, anti-IZUMO1 mAb can react with the sperm head even without permeabilization (Inoue et al . 2005). As depicted in Figure 1A, Hoechst 33342-stained sperm were incubated with cumulus-intact eggs for 10 min and subjected to immunostaining with an anti-IZUMO1 mAb without permeabilization of the sperm. Although the WT sperm underwent AR successfully, it failed to undergo AR in the eCs -deficient ( eCs -KO) sperm (73.9 ± 6.4% vs. 19.0 ± 2.8%; p = 0.001) (Figure 1B, C).
To induce sperm AR in both humans and mice (Buffone et al . 2014), the elevation of intracellular calcium in the post-acrosomal region is essential and is thought to be induced by the cyclic adenosine 3′,5′-monophosphate (cAMP) signaling pathway (Breitbart 2002). To assess the involvement of the cAMP-dependent pathway in the AR of eCs -KO sperm, we evaluated the AR in eCs -KO sperm after dibutyryl cAMP (dbcAMP), a cell-permeable synthetic analog of cAMP, treatment. As depicted in Figure 1D, sperm were collected from the cauda epididymis and incubated in dbcAMP (2, 3.5, and 5 μM) for 2 h without cumulus-intact eggs. The sperm were then immunostained with anti-IZUMO1 mAb and counterstained with 4’,6-diamidino-2-phenylindole (DAPI). AR is known to occur spontaneously, even when sperm are cultured without cumulus-intact eggs (Bhakta et al . 2019). The percentage of sperm that underwent AR among those incubated without cumulus-intact eggs was then evaluated. Consequently, without dbcAMP treatment, the percentage of acrosome-reacted (ARed) eCs -KO sperm was significantly lower than that of WT sperm (4.8 ± 2.1% vs. 20.5 ± 3.4%; p = 0.002; Figure 1F, left). After treatment with 2 μM dbcAMP, the percentage of ARed WT sperm remained unaltered, while the percentage of ARed eCs -KO sperm was strikingly elevated and turned out to be comparable with that of the WT sperm (17.1 ± 1.8% vs. 14.0 ± 1.3%; Figure 1F, right). Similarly, when the sperm were treated with 3.5 and 5 μM dbcAMP, the percentage of ARed WT sperm remained unaltered, while the percentage of ARed eCs -KO sperm was strikingly elevated but comparable with that of the WT sperm (15.5 ± 1.6% vs. 20.4 ± 1.1%; 17.6 ± 1.6% vs. 20.7 ± 1.6%). These results indicated that cAMP signaling plays an important role in the AR of eCs -KO sperm.
AR retardation in eCs -KO sperm was linked to decreased mitochondrial activity. Mitochondrial activity has been proposed to be a useful biomarker for evaluating the fertilization ability of sperm both in vivo and in vitro (Barbagallo et al . 2020). In particular, the regulation of sperm motility and AR is highly dependent on mitochondrial activity (Moraes and Meyers 2018). The activities of some mitochondrial enzymes such as CS and respiratory complexes showed a high correlation with sperm motility (Ruiz-Pesini et al . 2000), and asthenozoospermia was the cause of the reduction in their activities. As reported earlier (Kang et al . 2020), mitochondrial metabolic activity is altered by reduced amounts of citrates in eCs -KO sperm, suggesting a possible role for eCS proteins in regulating mitochondrial activity. However, eCs -KO sperm exhibited normal motility, indicating no correlation between eCS and sperm motility.
It is well known that ATP is essential for sperm motility and AR (Stival et al . 2016). As both glycolysis and OXPHO pathways can produce ATP in sperm (du Plessis et al . 2015), the activities of the enzymes involved in these pathways are important for ATP production. eCS is localized in the acrosome of the sperm head, while CS is localized inside the mitochondria of the sperm midpiece. Importantly, when eCs -KO sperm were incubated in the presence of dbcAMP, ARed sperm levels were significantly increased, raising the possibility that cAMP plays a critical role in eCs -KO sperm function. The cAMP-dependent protein kinase (PKA) signaling pathway plays a central role in the regulation of energy balance and metabolism (London et al . 2020). Because of its location, eCS may be directly or indirectly involved in ATP-related induction of AR. It would be interesting to uncover the mechanism by which eCS regulates energy metabolism in sperm.
In conclusion, the loss of eCS resulted in retarded AR, which was rescued by dbcAMP treatment, implying that eCS regulates AR via cAMP-mediated energy metabolism. Our results contribute to the understanding of eCS-mediated energy metabolism in sperm AR induction, which is widely observed in organisms, including humans.
Methods
Animals
Using the method reported in a previous study (Kang et al . 2020), mutant mice were generated from C57BL/6-derived embryonic stem cell clones by injecting blastocysts from C57BL/6 mice with genetically deleted Csl ( eCs ) (Csl tm1(KOMP)Vlcg ;MGI:4842999) obtained from the knockout mouse project (KOMP) repository, an NCRR-NIH-supported strain suppository. C57BL/6J mice (Japan SLC Inc., Shizuoka, Japan) were used as the controls. All the mice were housed under specific pathogen-free conditions. Food and water were provided ad libitum . All animal experiments were approved by the Institutional Animal Care and Use Committee of the National Research Institute for Child Health and Development (experimental number, A2004-004).
Antibodies and reagents
The rat anti-mouse IZUMO1 mAb required for immunostaining was kindly provided by Dr. Ikawa (Osaka University, Japan). Alexa 488- and 546-conjugated IgG were purchased from Molecular Probes (Invitrogen, Carlsbad, CA, USA) as secondary antibodies for immunohistochemistry. Nuclei were counterstained with DAPI (WAKO Pure Chemical Industries, Osaka, Japan). dbcAMP was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Sperm/cumulus penetration assay
Cauda epididymal sperm were incubated in a 200-μL drop of TYH medium containing Hoechst 33342 (2.5 µg/mL) for 10 min at 37 °C and then capacitated by incubation in a 100-μL drop of TYH medium for 2 h at 37 °C under 5% CO 2 . The capacitated sperm (150 sperm/µL) was mixed with the cumulus-oocyte complexes (COCs) in a 100-μL TYH drop and incubated for 10 min at 37 °C in 5 % CO 2 . The COCs and sperm were then transferred to a 100-μL drop of fresh TYH medium and fixed by treatment with 100-µL Hanks’ balanced salt solution containing 8% paraformaldehyde and 1% polyvinylpyrrolidone (PVP) for 15 min, followed by washing with HBS containing 0.5% PVP. COCs and sperm were immunostained with Alexa Fluor 488-labeled anti-IZUMO1 mAb. The COCs were lightly squashed to accommodate them within the 80-µm space between the glass slide and the coverslip and then observed under a fluorescent microscope (KEYENCE BZ-X710, Keyence, Osaka, Japan). Fluorescent images were captured as vertical sections with 3-μm intervals and then stacked into a single picture using BZ-analyzer software (Keyence), as described previously (Kang et al . 2010).
Spontaneous AR
Cauda epididymal sperm were dispersed in a 200-µL drop of albumin-free TYH medium for 10 min and subsequently incubated in albumin-containing TYH medium at 37 °C under 5% CO 2 . After 2 h of incubation in the absence and presence of 2, 3.5, and 5 mM dbcAMP, the sperm were transferred into a 1.5-mL microtube, washed with HBS, and fixed with 4% PFA in HBS on ice for 15 min. After fixation, the sperm were immunoreacted with an anti-IZUMO1 antibody, incubated with an Alexa Fluor 568-conjugated antibody against rabbit IgG, and counterstained with Hoechst 33342. Fluorescent images were obtained under a fluorescence microscope (KEYENCE BZ-X710).
Statistical analysis
Comparisons were made using one-way analysis of variance following Scheffe’s method, Mann–Whitney U -test, or Fisher’s exact test. Statistical significance was defined as p < 0.05. The results are expressed as the mean ± standard error of the mean.
Acknowledgments
Acknowledgments
this.convertHTML(ack)
Funding
This work was supported in part by JSPS KAKENHI (grant numbers JP19H01067 and JP19K09793).
References
- Barbagallo F, La Vignera S, Cannarella R, Aversa A, Calogero AE, Condorelli RA. 2020. Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility. J Clin Med 9: . [DOI] [PMC free article] [PubMed]
- Bhakta HH, Refai FH, Avella MA. 2019. The molecular mechanisms mediating mammalian fertilization. Development 146: . [DOI] [PubMed]
- Breitbart H. 2002. Intracellular calcium regulation in sperm capacitation and acrosomal reaction. Mol Cell Endocrinol 187: 139-44. [DOI] [PubMed]
- Buffone MG, Wertheimer EV, Visconti PE, Krapf D. 2014. Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim Biophys Acta 1842: 2610-20. [DOI] [PMC free article] [PubMed]
- du Plessis SS, Agarwal A, Mohanty G, van der Linde M. 2015. Oxidative phosphorylation versus glycolysis: what fuel do spermatozoa use? Asian J Androl 17: 230-5. [DOI] [PMC free article] [PubMed]
- Gervasi MG, Visconti PE. 2016. Chang's meaning of capacitation: A molecular perspective. Mol Reprod Dev 83: 860-874. [DOI] [PubMed]
- Iacobazzi V, Infantino V. Citrate--new functions for an old metabolite. Biol Chem. 2014 Apr 1;395(4):387–399}. doi: 10.1515/hsz-2013-0271. [DOI] [PubMed] [Google Scholar]
- Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005 Mar 10;434(7030):234–238}. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- Kang W, Harada Y, Yamatoya K, Kawano N, Kanai S, Miyamoto Y, Nakamura A, Miyado M, Hayashi Y, Kuroki Y, Saito H, Iwao Y, Umezawa A, Miyado K. Extra-mitochondrial citrate synthase initiates calcium oscillation and suppresses age-dependent sperm dysfunction. Lab Invest. 2019 Dec 19;100(4):583–595}. doi: 10.1038/s41374-019-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W, Zhou C, Koga Y, Baba T. 2010. Hyaluronan-degrading activity of mouse sperm hyaluronidase is not required for fertilization? J Reprod Dev 56: 140-4. [DOI] [PubMed]
- Moraes CR, Meyers S. 2018. The sperm mitochondrion: Organelle of many functions. Anim Reprod Sci 194: 71-80. [DOI] [PubMed]
- Ruiz-Pesini E, Lapeña AC, Díez C, Alvarez E, Enríquez JA, López-Pérez MJ. 2000. Seminal quality correlates with mitochondrial functionality. Clin Chim Acta 300: 97-105. [DOI] [PubMed]
- Stival C, Puga Molina Ldel C, Paudel B, Buffone MG, Visconti PE, Krapf D. 2016. Sperm Capacitation and Acrosome Reaction in Mammalian Sperm. Adv Anat Embryol Cell Biol 220: 93-106. [DOI] [PubMed]
- Surpin M, Chory J. The co-ordination of nuclear and organellar genome expression in eukaryotic cells. Essays Biochem. 1997;32:113–125}. [PubMed] [Google Scholar]
- Zhu Z, Li R, Wang L, Zheng Y, Hoque SAM, Lv Y, Zeng W. 2019. Glycogen Synthase Kinase-3 Regulates Sperm Motility and Acrosome Reaction via Affecting Energy Metabolism in Goats. Front Physiol 10: 968. [DOI] [PMC free article] [PubMed]