Abstract
Objectives
The aim of this study was to conduct a nationwide all comer description of incidence, contemporary management and outcome in Swedish spontaneous coronary artery dissection (SCAD) patients. The incidence of SCAD as well as the management and outcome of these patients is not well described.
Design
A nationwide observational study.
Participants and setting
All patients with SCAD registered in the Swedish Coronary Angiography and Angioplasty Register from 2015 to 2017 were included. The index angiographies of patients with registered SCAD were re-evaluated at each centre to confirm the diagnosis. Patients with non-SCAD myocardial infarction (MI) (n=32 601) were used for comparison.
Outcome measures
Outcomes included all-cause mortality, reinfarction or acute coronary reangiography.
Results
This study found 147 SCAD patients, rendering an incidence of 0.74 per 100 000 per year and a prevalence of 0.43% of all MIs. The average age was 52.9 years, 75.5% were women and 47.6% presented with ST-segment elevation MI. Median follow-up time for major adverse cardiac event was 17.3 months. Percutaneous coronary intervention was attempted in 40.1% of SCAD patients and 30.6% received stent. The use of antithrombotic agents was similar between the groups and there was no difference regarding outcomes, 10.9% vs 13.4%, p=0.75. Mortality was lower in SCAD patients, 2.7% vs 8.0%, p=0.03, whereas SCAD patients more often underwent acute reangiography, 9.5% vs 4.6%, p<0.01.
Conclusion
In this nationwide, all comer Swedish study, the overall incidence of SCAD was low, including 25% men which is more and in contrast to previous studies. Compared with non-SCAD MI, SCAD patients were younger, with lower cardiovascular risk burden, yet suffered substantial mortality and morbidity and more frequently underwent acute coronary reangiography.
Keywords: Myocardial infarction, Ischaemic heart disease, Coronary intervention
Strengths and limitations of this study.
All patients in Sweden considered having spontaneous coronary artery dissection (SCAD) during the study period, and all centres performing invasive coronary angiography are represented.
All angiographies where SCAD was reported in the Swedish Coronary Angiography and Angioplasty Registry were reviewed and validated by an independent interventional cardiologist.
All data regarding demographics, management, treatment and in-hospital outcomes are immediately registered on-line, thus limiting recall bias and missing values as these variables are compulsory to register.
Limitations include possible heterogeneity in the confirmation of SCAD diagnosis as the study did not include a core-lab.
Introduction
Spontaneous coronary artery dissection (SCAD) has been reported as the underlying cause of myocardial infarction (MI) in 0.2%–4% of all cases with an inherent risk of sudden cardiac death.1 2 The dissection occurs independently of atherosclerosis causing coronary flow obstruction and acute myocardial ischaemia.3 The majority of SCAD patients are women between 44 and 53 years.4 5 The presence of conventional cardiovascular risk factors is low.2 6 Instead, the aetiology of SCAD is multifactorial and often includes a pre-existing arteriopathy.2 7 8
SCAD presenting as Saw type 1 is an angiographic diagnosis, but as SCAD type 3 mimics atherosclerotic coronary artery disease (CAD) and type 2 is difficult to diagnose on angiography, clinical awareness, a high level of suspicion and sometimes intravascular imaging such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT) in these cases is needed.6 7 However, these procedures may lead to propagation of the dissection as guidewires can enter the false lumen.9 10
Percutaneous coronary intervention (PCI) also poses a risk of extending the dissection and carries a risk of stent malapposition subsequent to resorption of the intramural haematoma.1 4 5 9 11 12 Additionally, observational data indicate spontaneous healing within days to months after conservative treatment of SCAD.1 4 13 Hence, current recommendation emphasises conservative treatment of patients without ongoing large areas of ischaemia or haemodynamic instability.
The absence of randomised controlled trials (RCTs) leaves current guidelines based on expert opinion. While SCAD patients treated by PCI should receive standard dual-antiplatelet therapy (DAPT) the support for antiplatelet therapy in conservatively managed SCAD is lacking. Long-term mortality after SCAD has been reported to be low with survival rates between 92% and 100% after 3–6 years follow-up.4 14 On the other hand SCAD recurrence has been reported in 10%–17% during 3–4 years of follow-up.12 15
Although better recognised recently,16–18 SCAD still remains insufficiently studied as there are no nationwide reports on SCAD MIs relative to type 1 MIs, registered in the same period.
Thus, the aim was to study a Swedish all-comer MI population undergoing coronary angiography, describing incidence, prevalence, medical and invasive management and cardiovascular outcomes of SCAD compared with non-SCAD MI.
Method
Study population
This was a retrospective analysis of prospectively collected data using the Swedish Coronary Angiography and Angioplasty Registry (SCAAR).19 Between 17 December 2015 and 30 December 2017, all consecutive patients with recorded SCAD were identified using the SCAD variable launched in SCAAR on 15 December 2015. Patients with non-SCAD MI who underwent coronary angiography during the same time period were used for comparison.
SCAAR registry
The registry has previously been described, and covers 100% of patients undergoing coronary angiography and PCI in Sweden. Data on baseline characteristics, medical history, procedural characteristics and in-hospital complications are prospectively collected. SCAAR is a part of the Swedish Web-system for Enhancement and Development of Evidence based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART).19
Ongoing medication on arrival and at discharge were obtained by merging SCAAR with the Swedish register of information and knowledge about Swedish heart intensive care admissions (RIKS-HIA), another part of SWEDEHEART.
Angiographic SCAD diagnosis
All index angiographies of patients with SCAD were re-evaluated by an independent interventional cardiologist at each centre to confirm the diagnosis. Patients without confirmed SCAD were excluded. SCAD was defined according to the Saw angiographic classification of SCAD (online supplemental table 1). Coronary artery dissections evaluated as secondary to atherosclerotic plaque rupture or iatrogenic dissections were excluded.
bmjopen-2022-060949supp001.pdf (50.2KB, pdf)
Definition of outcomes and complications
The primary outcomes of this study were all-cause mortality, myocardial reinfarction and acute invasive coronary reangiography. Recurrent MI was defined as readmission according to the International Classification of Diseases codes I21 and I22. Acute reangiography was defined as an unplanned new coronary angiography after the index event. Information about all-cause mortality and MI were obtained by merging SCAAR with the national population registry and RIKS-HIA, respectively. Data about reangiography and PCI were derived from SCAAR. Follow up for death and MI was available until June 2018 and for coronary reangiography until January 2018.
Statistical analysis
Data are presented as numbers and percentages, mean±SD, or median with IQR, as appropriate. Comparisons of continuous variables were performed with the Student’ t-test, when normal distribution was present, otherwise the Mann-Whitney U test was used. Comparison of categorical variables between groups was performed using the χ2 test. Rate of cardiovascular events over time is presented using Kaplan-Meier curves and outcome comparisons were performed using the log-rank tests. Any p<0.05 is considered to indicate statistical significance. The overall proportion of missing data was low, <2.5% of patients regardless of variable, except for smoking status which was missing in 7.2% of patients with non-SCAD MI. IBM SPSS statistics V.25 was used.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Patient characteristics
In total, 264 patients from 30 centres were identified in SCAAR with an initial SCAD diagnosis alongside 32 601 patients with non-SCAD MI. After re-evaluating angiograms of all patients with registered definite or suspected SCAD, the diagnosis of definite SCAD was confirmed in 147 patients from 24 centres. According to Statistics Sweden, the average population in Sweden in the years 2015–2017 was 9 985 629 individuals, rendering an incidence of SCAD at 0.74 per 100 000 per year and a prevalence of 0.43% of all MI cases undergoing coronary angiography in Sweden at the same time. The prevalence of SCAD was 2.2% in the MI population <50 years, (7.3% and 0.8% in women and men, respectively).
With a mean age of 52.9 years, SCAD patients were younger than patients with non-SCAD MI, 68.5 year (p<0.01). The SCAD group consisted of 75.5% women compared with 31.9% of the non-SCAD MI group. The prevalence of cardiovascular risk factors, use of acetylsalicylic acid (ASA), statins and antihypertensive medications on admission was lower in the SCAD group (table 1).
Table 1.
Baseline characteristics in SCAD and non-SCAD MI
| SCAD MI n=147 n (%) |
Non-SCAD MI n=32 601 n (%) |
P value | |
| Age | 52.9±12.2 | 68.5±11.8 | <0.01 |
| Female gender | 111 (75.5) | 10 391 (31.9) | <0.01 |
| Diabetes | 3 (2.0) | 6921 (21.4) | <0.01 |
| Hypertension* | 39 (26.5) | 19 070 (59.4) | <0.01 |
| Hyperlipidaemia† | 20 (13.7) | 9125 (30.4) | <0.01 |
| Smoking history‡ | 56 (38.1) | 17 599 (58.1) | <0.01 |
| Previous MI | 16 (10.9) | 6733 (21.1) | <0.01 |
| Previous CABG | 0 | 1996 (6.1) | <0.01 |
| Previous PCI | 6 (4.1) | 5482 (16.8) | <0.01 |
| ACE-I or ARBs | 26 (17.7) | 11 904 (36.5) | <0.01 |
| Beta-blockers | 25 (17.1) | 10 107 (33.7) | <0.01 |
| ASA | 27 (18.5) | 8577 (28.6) | <0.01 |
| P2Y12-inhibitor | 4 (2.7) | 1642 (5.5) | 0.15 |
| DAPT | 3 (2.1) | 951 (3.2) | 0.44 |
| OAC | 7 (4.8) | 2426 (7.6) | 0.21 |
| Statins | 20 (13.7) | 9125 (30.4) | <0.01 |
*Antihypertensive treatment on admission.
†Treatment with lipid lowering agents on admission.
‡Active or previous smoking history.
ACE-I, ACE-inhibitor; ARB, angiotensin receptor blocker; ASA, acetyl salicylic acid; CABG, coronary artery bypass graft surgery; DAPT, dual antiplatelet therapy; MI, myocardial infarction; OAC, oral anticoagulants; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection.
Procedural characteristics
SCAD patients more often presented with STEMI when compared with non-SCAD MI patients, 47.6% and 39.3%, respectively (p<0.01). Coronary artery occlusion was found in 17.7% of SCAD patients and 23.8% of non-SCAD MI patients, p=0.08 (table 2). Coronary artery atherosclerosis was reported in 17 (11.6%) SCAD patients.
Table 2.
Coronary angiography, invasive and medical management in SCAD and non-SCAD MI
| SCAD n=147 n (%) |
Non-SCAD MI n=32 601 n (%) |
P value | |
| Coronary angiography, findings and procedures | |||
| STEMI | 70 (47.6) | 12 823 (39.3) | <0.01 |
| Coronary artery occlusion | 26 (17.7) | 7601 (23.8) | 0.08 |
| Conservative management | 88 (59.9) | 9493 (29.1) | <0.01 |
| Attempted PCI | 59 (40.1) | 23 108 (70.9) | <0.01 |
| PCI with stent | 45 (30.6) | 21 455 (65.8) | <0.01 |
| OCT/IVUS | 36 (24.5) | 1260 (3.9) | <0.01 |
| General success* | 51 (86.4) | 21 913 (94.8) | <0.01 |
| Medical therapy at discharge | |||
| ACE-I or ARBs | 87 (59.2) | 24 187 (74.2) | <0.01 |
| Beta-blockers | 118 (81.9) | 25 370 (86.0) | 0.16 |
| ASA | 134 (93.1) | 26 522 (89.9) | 0.21 |
| ASA only | 17 (11.6) | 3096 (9.5) | 0.39 |
| P2Y12-inhibitor | 123 (85.4) | 24 871 (84.3) | 0.72 |
| DAPT | 117 (81.3) | 23 418 (79.4) | 0.59 |
| OAC | 13 (8.8) | 3935 (12.2) | 0.22 |
| Statins | 110 (76.4) | 27 036 (91.7) | <0.01 |
*Subjective assessment by the operator. The operator has reached the main aim of the treatment.
ACE-I, ACE-iInhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; DAPT, dual-antiplatelet therapy; MI, myocardial infarction; OAC, oral anticoagulant; OCT/IVUS, optical coherence tomography/intravascular ultrasound; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection; STEMI, ST-segment elevation myocardial infarction.
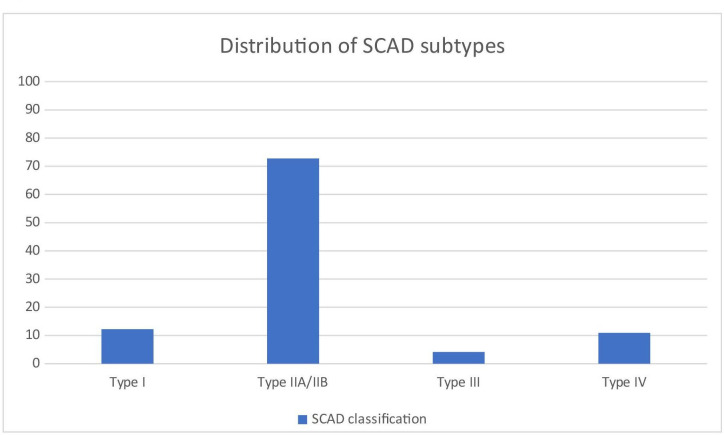
Type 1 dissection was found in 12.2%, type 2A/2B dissection in 72.8%, type 3 dissection in 4.1% and type 4 in 10.9% of SCAD patients (figure 1). Intracoronary imaging, OCT/IVUS, was used in 24.5% of SCAD patients and in 3.9% of non-SCAD MI patients.
Figure 1.
Distribution of SCAD subtypes. SCAD, spontaneous coronary artery dissection.
PCI was attempted in 40.1% of SCAD patients and 30.6% received stent. In non-SCAD MI patients corresponding figures were 70.9% and 65.8%, respectively.
SCAD patients with 100% coronary artery occlusion underwent PCI of which 65.5% were treated with stent implantation. Patients with non-occlusive SCAD were treated with stent implantation in 23% of cases. Intracoronary imaging was used in 24.5% of SCAD-procedures compared with 3.3% in non-SCAD MI. The general success of PCI was 86.4% in the SCAD group compared with 94.8% in the non-SCAD MI population, p<0.01. (table 2)
Management stratified by type of dissection is presented in online supplemental table 2.
Inpatient care time and medical treatment at discharge
There was no difference in days of hospitalisation during index event between the two groups, with a median of 4 days, p=0.93.
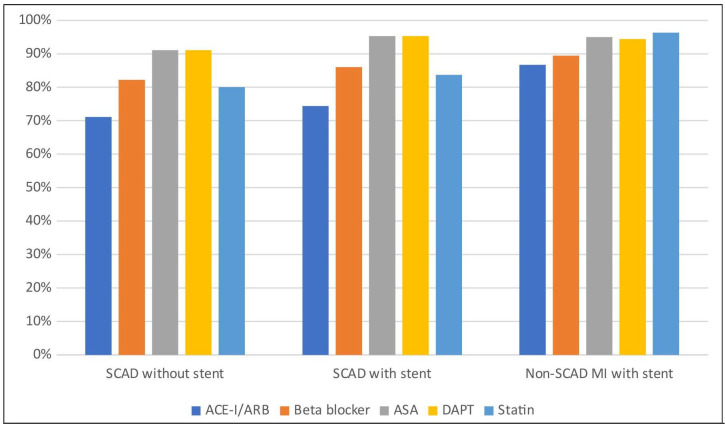
The use of betablockers, ASA, P2Y12-inhibitors, DAPT and oral anticoagulants was similar between the groups at discharge. SCAD patients received DAPT in 81.3% while 11.6% received ASA only and 2.7% received no antiplatelet therapy. Non-SCAD MI patients received more often ACE-I/ARBs and statins at discharge, yet statins were prescribed in 76.4% of SCAD cases (table 2, figure 2).
Figure 2.
Medical therapy at discharge. ACE-I, ACE-inhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; DAPT, dual antiplatelet therapy; MI, myocardial infarction; SCAD, spontaneous coronary artery dissection.
Outcomes
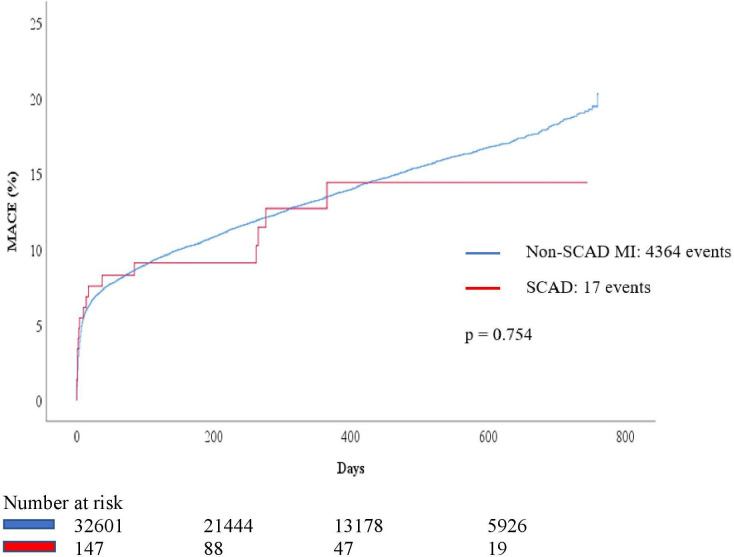
Median follow-up time for major adverse cardiac events (MACE) was 17.3 months. There was no difference in rate of combined outcomes between the SCAD and the non-SCAD MI groups (10.9% and 13.4%, p=0.75) (table 3, figure 3).
Table 3.
Outcome in SCAD and non-SCAD myocardial infarction
| SCAD MI n=147 n (%) |
Non-SCAD MI n=32 601 n (%) |
P value | |
| Death | 4 (2.7) | 3099 (9.7) | <0.01 |
| MI | 3 (2.0) | 1424 (4.4) | 0.20 |
| Acute coronary re-angiography after discharge | 14 (9.5) | 1495 (4.6) | <0.01 |
MI, myocardial infarction; SCAD, spontaneous coronary artery dissection.
Figure 3.
Outcomes in SCAD and non-SCAD MI. MACE, major adverse cardiac events; MI, myocardial infarction.; SCAD, spontaneous coronary artery dissection.
Outcomes in SCAD subtypes are presented in online supplemental table 3.
Median number of days to outcomes was 10 for SCAD and 25 for non-SCAD MI. The rate of all-cause mortality in SCAD (2.7%) was lower when compared with the non-SCAD MI population (9.7%) (p<0.01). There was no difference in the rate of reinfarction between the SCAD (2.0%) and the non-SCAD MI population (4.4%) (p=0.20). Median number of days until reinfarction in SCAD patients was 37 days. Median time to acute reangiography was 12 (IQR: 127.5) days in the SCAD group and 14 (IQR: 208) days in the non-SCAD group which was statistically non-significant. The SCAD population was more often subject to acute reangiography after the index event (9.5%) than the non-SCAD MI population (4.6%) (p<0.01) (table 3).
Discussion
In this study with 100% nationwide coverage of MI patients undergoing coronary angiography during a 2-year period, we found an incidence of SCAD at 0.74 cases per 100.000 inhabitants per year and a SCAD prevalence of 0.43% of all MI cases in Sweden at the time. The prevalence of SCAD in the MI population <50 years was 2.2% and 7.3% of MI cases in women <50 years. We found an equally high rate of combined outcomes and recurrent MI in the two MI groups whereas SCAD patients were more often subject to acute coronary reangiography. Although 59.9% were treated conservatively without PCI or CABG, 81.3% of SCAD patients were discharged with DAPT.
Epidemiology
While the prevalence of SCAD was lower than most other studies have suggested, we found a higher prevalence of SCAD than previously published multicentre studies.20–22 An older study of 32 869 patients from 3 centres in western Denmark identified a SCAD prevalence of 0.2% during 8 years whereas a Japanese study of 20 195 MI patients collected in 20 centres during 13 years identified a SCAD prevalence of 0.31%.20–22 Our results are in concordance with the recently published meta-analysis by Franke et al23 including more than 2000 patients. In addition, we did not exclude patients with angiographic signs of atherosclerosis in other than SCAD vessels. On the other hand, when comparing with smaller single-centre studies, the prevalence in our cohort is lower. This might be attributed to the relatively new SCAD variable in SCAAR with an increasing learning curve among interventionists to recognise and report all types of SCAD in the SCAAR registry. Thus, a certain underdiagnosis may have caused a lower degree of identification of SCAD cases than in centres with special interest in SCAD.
We also found a lower prevalence of SCAD in female MI patients <50 years (7.3%) compared with previous studies. Four studies have reported a SCAD prevalence of 23%–36% in women below 50–60 years with MI. Three of these are small single centre studies including ≤20 SCAD cases less than 60 years.1 20 21 The fourth by Nakashima et al reported a SCAD prevalence of 35% in women <50 years with MI.11 This is in contrast to our findings and we speculate it to be related to genetic variations and a low prevalence of CAD in Japan.
Risk factors
Our results are in line with previous studies showing that SCAD predominantly affects middle-aged women with a low prevalence of cardiovascular risk factors and ongoing cardiovascular medications.1 2 4 9 11 12 14 18 20 Interestingly, 10% of SCAD cases included in our study had suffered a previous MI. This may be explained by SCAD recurrency as we do not know if the indexed SCAD occasion was the first. Rate of recurrency has been described to be between 4.7% and 17% in 2–4 years which aligns with our findings.11 12 14 Other possible explanations are inclusion of patients with concurrent atherosclerosis in other coronary segments than the one affected by SCAD.
Sex
Our SCAD population included 25% men which is in contrast to previous studies—in particular to those where SCAD patients with atherosclerosis have been excluded.1 9 11 However, there are studies with a proportion of male patients between 23% and 46.2%.21 22 A consequence of excluding all SCAD patients with any atherosclerosis is the selection of younger and female patients, with a low burden of concomitant co-morbidity. When describing findings from imaging, genetic or proteomic studies there could be a rationale to select patients with a clear-cut SCAD diagnosis. On the other hand, when describing incidence, prevalence, management and prognosis, it is of great importance not to introduce a selection bias by excluding patients with concomitant atherosclerotic manifestations. The current study included all patients with SCAD unless iatrogenic or due to plaque rupture and hence describes the entire SCAD population without selection. Our results indicate that also men are, to a larger extent than previously thought, affected by SCAD, and that the diagnosis should not be overlooked but sought after in these patients too.
Angiography and intervention
Type 2 dissection was the most common angiographic manifestation, in accordance with previous studies, followed by type 1, however, only seen in 12.2% as opposed to 29%–55% in previous reports. Meanwhile, the prevalence of type 3 was similar between this and other studies.1 4 9 11 This indicates that intimal flap appearance and dual lumen sign is less prevalent than previously suggested, probably due to increasing recognition of non-classical appearance of SCAD. Although intravascular imaging was more widely used in SCAD patients, a majority of type 3s and 4 SCAD cases were diagnosed without using OCT/IVUS. As type 3 is defined as angiographically indistinguishable from atherosclerotic CAD, diagnosing type 3 without intravascular imaging is a limitation in this study.
There are several feasible reasons for this, including technical difficulties in the case of distal occlusions or ignorance of its necessity. In addition, the diagnosis of SCAD mimicking CAD does not always require intravascular imaging but can be made with enough experience without arduous catheterisation.
SCAD patients underwent PCI and stenting less frequently at the index event than non-SCAD MI patients, although PCI was attempted in 40% of cases and 30% received stents. Other retrospective studies of SCAD patients have reported revascularisation rates between 12% and 56%.4 5 9 11 24 As there are no RCTs describing optimal management, we cannot comment on overtreatment or undertreatment. Nor do we have information regarding the clinical circumstances underlying choice of treatment, for example, PCI on vital indication in haemodynamically unstable patients.
Medication
We found the medication at discharge to be remarkably similar in patients with SCAD and non-SCAD MI, including the use of ASA, P2Y12-inhibitors and DAPT. This might reflect adherence to current guidelines for ACS in the absence of SCAD specific evidence.6 7 In this study, 80% of SCAD patients were treated with betablockers, which has been proposed to be beneficial.15 In a retrospective study of 327 patients the use of betablockers was associated with a lower risk of recurrent SCAD and this therapy could therefore be considered.6 7 The prescription rate of statins was high in SCAD patients, despite not being recommended.6 7 Our findings thus reflect the lack of familiarity that most cardiologists may have had with managing SCAD, especially prior to 2018, which is the period when our patients were included.
Outcomes
The overall rate of outcomes did not differ between SCAD and non-SCAD MI. However, all-cause mortality was lower in SCAD, yet the rate of recurrent MI was equal. Furthermore, SCAD patients were more often subject to acute coronary re-angiography after the index event, 9.5%. This is evidence of significant morbidity in SCAD, especially as age and cardiovascular risk factors have not been adjusted for, due to the relatively small study population with SCAD. In three recently published European SCAD-studies16–18 the unplanned reangiography was 4%, 8.5% and 5.3%, respectively. This study found a death rate of 2.7% after a median follow-up of 17.3 months indicating a higher mortality than in previous studies. This could be caused by inclusion of a more representative and unselected population of SCAD patients.
Although recurrent MI was equal between the two groups, our 2% recurrency rate is lower than previously described, varying between 4.8% and 12% per year.11 15 SCAD recurrency has been reported at 4.7%–17% in 22–47 months.11 12 14 The discrepancy between our study and the American and Canadian series is however small and may be due to different lengths of follow-up as adverse events may not be evenly distributed in time.12 15
The cause of the high rate of acute, unplanned coronary reangiography after the index event is not known to us and is not explained by recurrent MI or need for revascularisation as PCI was attempted in only 5/14 acute coronary reangiographies. Further studies are needed to elucidate this, although it is plausible that a high prevalence of recurrent angina and difficulties in chest pain risk assessment could be contributing factors.
Strengths and limitations
This study has identified all patients in Sweden considered having SCAD during the study period, and all centres performing invasive coronary angiography are represented. All angiographies where SCAD has been reported in the SCAAR registry have been reviewed and validated by an independent interventional cardiologist. All data regarding demographics, management, treatment and in-hospital outcomes are immediately registered on-line, thus limiting recall bias and missing values as these variables are compulsory to register.
Limitations of this study include possible heterogeneity in the confirmation of SCAD diagnosis as the study did not include a core-lab. Segment distribution in SCAAR in angiography alone is not compulsory, therefore, it is missing information in many SCAD patients. A segment analysis was not done. The occurrence of FMD was not available. Additionally, data that could not be derived from angiographic re-evaluation was derived from registries. Predictors of MACE were not analysed in this small population.
Conclusion
SCAD patients were comparatively young and previously healthy, yet suffered substantial mortality and morbidity and are frequently subject to acute coronary reangiography and its accompanying risks. As both incidence and prevalence are low, data highlight the careful need of diagnostic awareness in both men and women and in patients with coexisting atherosclerotic CAD.
Supplementary Material
Footnotes
Contributors: HW, SSL, DV and ES are responsible for the conception and design of the study, have full access to all data, analysed and interpreted the data and drafted the manuscript. CP gathered the PCI data, critically revised the manuscript and added important intellectual content. CD, LJ, NJ, TK, PT and TY critically revised the manuscript and added important intellectual content. ES accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish
Funding: This work was supported by Östergötland County Council (LIO-701071, E.S.) and the Swedish Heart and Lung foundation (nr. 20190379, E.S).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data are available from the corresponding author on request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All patients were informed of their participation in the SCAAR registry, and their possibility to withdraw their consent at any time. Anyhow, according to Swedish regulations, written informed consent is not required for registration in national quality registries such as SCAAR. Permission for the study was obtained from the regional Ethical Review Board, Linkoping, Sweden (Dnr 2018/122-31), and complied with the Declaration of Helsinki. Patient data were anonymised to protect integrity.
References
- 1.Rashid HNZ, Wong DTL, Wijesekera H, et al. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome--A single-centre Australian experience. Int J Cardiol 2016;202:336–8. 10.1016/j.ijcard.2015.09.072 [DOI] [PubMed] [Google Scholar]
- 2.Saw J, Ricci D, Starovoytov A, et al. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013;6:44–52. 10.1016/j.jcin.2012.08.017 [DOI] [PubMed] [Google Scholar]
- 3.Maehara A, Mintz GS, Castagna MT, et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol 2002;89:466–8. 10.1016/S0002-9149(01)02272-X [DOI] [PubMed] [Google Scholar]
- 4.Rogowski S, Maeder MT, Weilenmann D, et al. Spontaneous coronary artery dissection: angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter Cardiovasc Interv 2017;89:59–68. 10.1002/ccd.26383 [DOI] [PubMed] [Google Scholar]
- 5.Tweet MS, Eleid MF, Best PJM, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014;7:777–86. 10.1161/CIRCINTERVENTIONS.114.001659 [DOI] [PubMed] [Google Scholar]
- 6.Adlam D, Alfonso F, Maas A, et al. European Society of cardiology, acute cardiovascular care association, SCAD Study group: a position paper on spontaneous coronary artery dissection. Eur Heart J 2018;39:3353–68. 10.1093/eurheartj/ehy080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes SN, Kim ESH, Saw J, et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American heart association. Circulation 2018;137:e523–57. 10.1161/CIR.0000000000000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persu A, Giavarini A, Touzé E, et al. European consensus on the diagnosis and management of fibromuscular dysplasia. J Hypertens 2014;32:1367–78. 10.1097/HJH.0000000000000213 [DOI] [PubMed] [Google Scholar]
- 9.Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014;7:645–55. 10.1161/CIRCINTERVENTIONS.114.001760 [DOI] [PubMed] [Google Scholar]
- 10.Prakash R, Starovoytov A, Heydari M, et al. Catheter-Induced Iatrogenic Coronary Artery Dissection in Patients With Spontaneous Coronary Artery Dissection. JACC Cardiovasc Interv 2016;9:1851–3. 10.1016/j.jcin.2016.06.026 [DOI] [PubMed] [Google Scholar]
- 11.Nakashima T, Noguchi T, Haruta S, et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: a report from the angina Pectoris-Myocardial infarction multicenter Investigators in Japan. Int J Cardiol 2016;207:341–8. 10.1016/j.ijcard.2016.01.188 [DOI] [PubMed] [Google Scholar]
- 12.Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579–88. 10.1161/CIRCULATIONAHA.112.105718 [DOI] [PubMed] [Google Scholar]
- 13.Main A, Prakash R, Starovoytov A, et al. Characteristics of extension and de novo recurrent spontaneous coronary artery dissection. EuroIntervention 2017;13:e1454–9. 10.4244/EIJ-D-17-00264 [DOI] [PubMed] [Google Scholar]
- 14.Lettieri C, Zavalloni D, Rossini R, et al. Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol 2015;116:66–73. 10.1016/j.amjcard.2015.03.039 [DOI] [PubMed] [Google Scholar]
- 15.Saw J, Humphries K, Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017;70:1148–58. 10.1016/j.jacc.2017.06.053 [DOI] [PubMed] [Google Scholar]
- 16.Mori R, Macaya F, Giacobbe F, et al. Clinical outcomes by angiographic type of spontaneous coronary artery dissection. EuroIntervention 2021;17:516–24. 10.4244/EIJ-D-20-01275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Combaret N, Gerbaud E, Dérimay F, et al. National French registry of spontaneous coronary artery dissections: prevalence of fibromuscular dysplasia and genetic analyses. EuroIntervention 2021;17:508–15. 10.4244/EIJ-D-20-01046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Guimaraes M, Bastante T, Macaya F, et al. Spontaneous coronary artery dissection in Spain: clinical and angiographic characteristics, management, and in-hospital events. Rev Esp Cardiol 2021;74:15–23. 10.1016/j.rec.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 19.Jernberg T, Attebring MF, Hambraeus K, et al. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart 2010;96:1617–21. 10.1136/hrt.2010.198804 [DOI] [PubMed] [Google Scholar]
- 20.Vanzetto G, Berger-Coz E, Barone-Rochette G, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg 2009;35:250–4. 10.1016/j.ejcts.2008.10.023 [DOI] [PubMed] [Google Scholar]
- 21.Mortensen KH, Thuesen L, Kristensen IB, et al. Spontaneous coronary artery dissection: a Western Denmark heart registry study. Catheter Cardiovasc Interv 2009;74:710–7. 10.1002/ccd.22115 [DOI] [PubMed] [Google Scholar]
- 22.Nishiguchi T, Tanaka A, Ozaki Y, et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2016;5:263–70. 10.1177/2048872613504310 [DOI] [PubMed] [Google Scholar]
- 23.Franke KB, Nerlekar N, Marshall H, et al. Systematic review and meta-analysis of the clinical characteristics and outcomes of spontanous coronary artery dissection. Int J Cardiol 2021;322:34–9. 10.1016/j.ijcard.2020.08.076 [DOI] [PubMed] [Google Scholar]
- 24.Alfonso F, Paulo M, Lennie V, et al. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a "conservative" therapeutic strategy. JACC Cardiovasc Interv 2012;5:1062–70. 10.1016/j.jcin.2012.06.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-060949supp001.pdf (50.2KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data are available from the corresponding author on request.