Abstract
Background:
To evaluate efficacy, pharmacokinetics(PK) and pharmacodynamics (PD) of single-agent everolimusin pediatric patients with radiographically progressive low-grade glioma (LGG).
Methods:
Everolimus was administered at 5 mg/m2 once daily as a tablet or liquid for a planned 48-week duration or until unacceptable toxicity or disease progression. Patients with neurofibromatosis type 1 were excluded. PK and PDendpoints were assessed in consenting patients.
Results:
Twenty-three eligible patients (median age: 9.2 years) were enrolled. All patients received prior chemotherapy(median number of prior regimens: 2)and/orradiotherapy (two patients). By week 48, two patients had a partial response, 10stable disease, and 11 clinical or radiographic progression; two discontinued study prior to one year (toxicity: 1, physician determination:1). With a median follow-up of 1.8 years (range: 0.2–6.7 years), the 2, 3, and 5-year PFS were 39±11%, 26±11%, and 26±11%, respectively; two patients died of disease. The 2, 3 and 5-year OS were all 93±6%. Grade 1 and 2 toxicities predominated; two definitively related grade 3 toxicities (mucositis and neutropenia) occurred. Grade 4 elevation of liver enzymes was possibly related in one patient. Pre-dose blood levelsshowed substantial variability between patientswith 45.5% below and 18.2% above the target range of 5–15 ng/mL.PD analysis demonstrated significant inhibition in phospho-S6, 4E-BP1 and modulation of c-Myc expression.
Conclusion:
Daily oral everolimusprovides a well-tolerated, alternative treatment for multiply recurrent, radiographically progressive pediatric LGG. Based on these results, everolimus is being investigated further for this patient population.
Keywords: Clinical Trial, Phase 2, mTOR, Brain Tumor, Low-grade glioma, everolimus
Introduction
Low-grade gliomas (LGGs) are the most common tumor of the central nervous system (CNS) in children and comprise a number of histologic subtypes 1. While pediatric patients with low-grade gliomas have longer survival compared to patients with other CNS tumors,2 the unique biology of these lesions results in numerous recurrences or progressions for many patients, necessitating additional therapies and consequently cumulative toxicities.3 Complete surgical resection can be curative but the tumor’s location or infiltrative nature often makes this infeasible. For incompletely resected LGGs, radiation therapy was previously considered standard treatment but fell out of favor due to significant long-term toxicity with respect to neurocognitive impairment, endocrine dysfunction, secondary tumor risk and stroke.2Thus, many centers attempt to defer irradiation with the use of chemotherapy, even for older children and adolescents.3–6 Moreover, no standard therapy regimen exists for relapsed disease although multiple regimens have been evaluated.7–12
The mammalian target of rapamycin (mTOR) serves as a pivotal signaling pathway regulating key cellular processes, including metabolism, protein synthesis, cell cycle progression, angiogenesis, and apoptosis and autophagy.13,14Patients with Tuberous Sclerosis (TS) have genetic alterations in Tsc1/2 and exhibit dysregulation of mTOR/S6 kinase signaling, resulting in the development of subependymal giant-cell astrocytomas. Everolimus, a macrotide derivative of rapamycin that selectively inhibits mTOR, is highly active in inducing regression of TS-associated SEGA and had been previously approved for that therapeutic indication.15–17Both neurofibromatosis type 1 (NF1)-associated and sporadic pediatric LGGs have demonstrated abnormal signaling upstream of mTOR through mutations in receptor tyrosine kinases, or more commonly for sporadic LGG, through alterations in BRAF.18–21 Given the well-tolerated toxicity profile of everolimus and central role of the Ras/Raf/mTOR pathways in pediatric LGGs,22 we evaluated its activity in patients with radiographically progressive disease.
Patients and Methods
Patients, ages 3–21 years, without NF1 or TS and with confirmed LGGhistologiesdefined as a World Health Organization grade 1 pilocytic astrocytoma; grade 2 pilomyxoid, fibrillary, protoplasmic or mixed astrocytoma; pleomorphic xanthoastrocytoma; infantile desmoplastic astrocytoma; ganglioglioma; oligodendroglioma; or mixed oligo-astrocytoma, were eligible. Separate trials for NF1-associated LGG or TS-associated SEGA were available andthus those patients excluded. Evidence of radiographically progressive disease with at least one measurable lesion after at least one prior cancer-directed regimen (irradiation or chemotherapy with no upper limit of prior therapies) was required. Clinical symptom progression alone, such as deterioration of vision, was not adequate for trial entry. Lansky or Karnofsky performance score ≥50% was required. Patients had to have recovered from prior therapies and could not be taking strong inducers or inhibitors of CYP3A to avoid potential confounding factors based on prior conflicting studies suggesting patients with certain genetic polymorphismsmay or may not require higher doses of drug to achieve adequate trough concentrations with rapamycin-based therapies.23, 24At least 4 weeks from prior chemotherapy (6 weeks if it included a nitrosourea), 6 months from radiation therapy and at least 2 weeks or five half-lives, whichever was longer, for biologic agents were required for eligibility. Physiologic steroid and non-enzyme inducing anticonvulsants were permitted. Patients were required to have adequate organ function defined as an absolute neutrophil count (ANC)≥1,000/uL, platelets ≥100,000/uL, serum creatinine no more than the upper limit of normal for age (ULNFA), bilirubin ≤1.5X ULNFA, transaminases ≤2.5X ULNFA, a serum albumin >2g/dL and an INR of <1.3. Patients were also required to have a fasting LDL cholesterol ≤ ULNFA, a fasting serum cholesterol ≤300mg/dL or ≤7.75mmol/L and a fasting triglyceride of ≤2.5X ULNFA. Exclusion criteria included chronic, systemiccorticosteroids or other immunosuppressive agents (topical or inhaled corticosteroids allowed), severe and uncontrolled underlying medical conditions, pregnant or breast-feeding females, patients with gastrointestinal malabsorption conditions,patients previouslytreated with another mTOR inhibitor, and patients with prior documented hepatitis B or C infection.
This protocol (NCT00782626), conducted under IND 104003,was approved by Novartis, the POETIC Consortium operations center at Memorial Sloan Kettering Cancer Center and the Dana-Farber/Boston Children’s Hospital Institutional review board as well as those of all participating sites. All patients or their legal guardians provided written informed consent and/or assent as appropriate at enrollment.
Everolimuswas administered as a tablet or oral liquid at a standard dose of 5mg/m2once daily, either fasting or after a light, fat-free meal, in 28-day cycles for a planned duration of 12 cycles (48 weeks). Dose modifications were based on the CTCAEv3.0 criteria (http://ctep.cancer.gov/forms/CTCAEv3.pdf) except for hyperlipidemia and pneumonitis. Grade 2 toxicities permitted dose interruption followed by retreatment at full dose, while grade 3 toxicities resulted in treatment interruption followed by a reduction to a lower dose level (dose level −1: 3mg/m2; dose level −2:2mg/m2). Hyperlipidemia was treated using diet and medical management with lipid lowing agents rather than discontinuing treatment. Grade 2 non-infectious pneumonitis required dose interruption and possible addition of systemic steroids with reinstitution of therapy at a lower dose level, while grade 3 non-infectious pneumonitis required therapy discontinuation. The management algorithms for stomatitis and non-infectious pneumonitis are provided in the supplemental data (see Supplementary Table S1). For hematologic toxicities, the following criteria were used: (1) Platelets ≥50,000/uL and <75,000/uL required a dose interruption until recovery to ≤grade 1 at which point everolimus was restarted without dose reduction. If the toxicity recurred, subsequent therapy was reduced by one dose level; (2) Platelets ≥25,000/uL and <50,000/uL required dose interruption until ≤grade 1, and everolimus was resumed at one dose level below. Recurrent grade 3 thrombocytopenia resulted in therapy discontinuation. Grade 4 thrombocytopenia required discontinuation of treatment; (3) for an ANC between ≥500/ul and <1,000/uL, everolimus was held until ANC recovery to ≤grade 2 at which point everolimus was restarted at full dose. If toxicity recurred, everolimus was held until recovery to ≤grade 2 and restarted at one dose level below. If toxicity recurred, everolimus was discontinued. For grade 4 ANC, everolimus was held until recovery to ≤grade 2, then restarted at a lower dose; and if it recurred, everolimus was discontinued. For febrile neutropenia with ANC grade 3, everolimus was held until afebrile and ANC recovery to ≤grade 2. The dose was then reduced by one level. If febrile neutropenia recurred, everolimus was discontinued. For grade 4 febrile neutropenia, everolimus was discontinued.
To assess the pharmacokinetic profile of everolimus in children with LGG,everolimuswhole blood concentrations were centrally determined by a validated high performance liquid chromatography tandem mass spectroscopy assay with stable-isotope dilution analysis. The inter-assay imprecision of the method was 15.4%, 10.7%, 6.9% and 6.4% expressed as % coefficient of variation for the quality control samples at the lower limit of quantification (1 ng/mL), the low, medium and high concentrations, respectively. Correspondingly, the intra-assay imprecision was 3.6%, 11.1%, 3.96% and 4.1%, respectively. Blood everolimus concentrations were determined pre-dose, and at 2 and 5 hours post-dose. Pharmacokinetic analysis was performed using MW/Pharm clinical software (version 3.82, Mediware, Prague, Czech Republic). Pharmacokinetic parameter estimates were generated using a previously published two-compartment pediatric PK model as the Bayesian prior.25Pharmacokinetic parameter estimates such as clearance and volume of distribution were allometrically scaled to body weight to account for body size differences among patients.26,27True trough concentration at 24 hours post-dose and area under the concentration-time curve (AUC) for 24 hours of dosing interval were generated for each individual patient.
Pharmacodynamic analysis was also undertaken in consenting patients. Whole blood (2 mL) was obtained from study patients in EDTA tubes prior to initiating therapy on day 7 (+/− 3) and day 14 (+/− 3) of course 1, prior to start of courses 3, 5, 7, 9, and at completion of treatment (end of course 12). A total of 8 samples were drawn when possible from each patient before everolimus administration and on treatment days 15, 28, and 62. Peripheral blood mononuclear cells (PBMCs) were isolated from each sample using the Ficoll reagent and stored at −80°C prior to analysis. Total protein was analyzed by Western blotting for levels of phospho-p70 S6 kinase (Thr389), p70 S6 kinase, phospho-S6 ribosomal protein (Ser235/236), S6 ribosomal protein, phospho-4E-BP1 (Thr70), and c-Myc (9E10): sc-40. Antibodies were obtained from Cell Signaling Technology, Inc., and Santa Cruz Biotechnology. Preliminary correlations of PK with changes in pharmacodynamics parameters including p70s6 kinase activity in peripheral blood mononuclear cells were evaluated as previously reported in other populations treated with mTOR inhibitors.25, 28, 29
Response evaluation
All patients were evaluated by MRI within 21 days prior to treatment initiation; progressive disease was defined based on comparing scans from baseline to that demonstrating best response. On-therapy imaging was performed after course 1, every three courses thereafter, and at completion of therapy. All imaging underwent blinded central radiographic review. Due to the complexity of pediatric LGG appearance on MRI and in keeping with the guidelines set forth by the POETIC Consortium, tumor response was not strictly determined by change in enhancement on post contrast T1 images; measurable change in size and extent of the target lesion(s) was required on fluid-attenuated inversion recovery/T2 sequences or on pre-contrast T1 images. The decision to evaluate stable disease (SD) within the response criteria reflects the resultant tumor stabilization for pediatric LGGs that may occur with inhibition of the AKT pathway, as hadbeen observed with other chemotherapy regimens. Response for target lesions was based on 3 dimensions with an elliptical model volume used (0.5L*W*T; L: tumor extent in plane perpendicular to the selected plane; W: longest measurement of the tumor width; T: transverse measurement perpendicular to the width). Complete responses (CR) represented disappearance of all target lesions and no new lesions. Partial responses (PR)correlated to>/= 65% decrease in sum of the products from baseline. Progressive disease correlated to 40% or more increase in any target lesion (referent smallest product observed on therapy). Stable disease was neither sufficient decrease nor increase to meet other criteria. While radiographic progressive disease was required for study entry (symptom progression was not sufficient), development of clinical progression even in the context of stable disease on MRI resulted in removal of patient from protocol therapy and was considered progressive disease. Off-therapy scans were performed as per institutional standard.
Statistics
The primary objective of the protocol was to determine if treatment demonstrated a response rate ≥25%, which would be considered promising for further study. A response rate <5% was considered evidence of an inactive regimen. A minimum of twenty patients with evaluable radiographic progressive LGG was required. Assuming 15% of patients might not be evaluable or eligible, 23 patients were required for enrollment. Centrally reviewed response assessment was based on the presence of a CR, PR or SD after completion of therapy and included patients’ responses for those who came off treatment early for toxicity. A patient’s best response was utilized;patients who demonstrated PD within the 12 cycles of protocol therapy were counted as PDfor statistical analysis, even if they had initially responded. A one-stage design was selected assuming the response rate was likely to occur slowly given the biologic nature of these tumors. If at least 3 responders were present among the 20, then everolimus would be considered promising for future studies. If the true response rate was 25%, the chance of concluding that the treatment was active would be 0.91 (power), with type I error rate 0.08.
Bioanalytical analysis of PK samples was conducted using validated assays with samples from 22 of 23 patients. Plasma concentrations were summarized by descriptive statistics, including mean, standard deviation, coefficient of variation, minimum, maximum, and median. Pharmacokinetic parameters, including clearance, volume of distribution, pre-dose trough (Ctrough), and AUC were evaluated. Correlations of response with changes in pharmacodynamic parameters including inhibition of p70s6 kinase activity, 4E-PB1 and c-Myc in PBMCs were performed. Toxicity of everolimus was descriptive. Tumor tissue assessment of mTOR targets included assessment ofpS6, p4EPB1 and pEIF4G expression. A companion biology protocol (PI Karajannis) was developed to obtain tumor material, if available, using immunohistochemical staining intensity (graded 0–3) as previously described.30Blinded analysis was then performed on the samples.
Results
This protocol accrued 23 eligible and evaluable patients between September 2009 and September 2011. Characteristics of the patients are provided in Table 1 with 17 females (74%),6 males (26%) and an age range of 3.8 to 17.1 years (median age, 9.2 years). The median age at initial diagnosis was 4.3 years (range: 0.3–11.8 years). Median body surface areaat study entry was 1 m2 (range: 0.6–1.62m2). All patients had previously received a chemotherapy-containing regimen (median number of prior treatment regimens 2) and 2 had received prior radiation therapy. Performance status at trial entry ranged from 60–100% (median 90%). Patients received from 2–12 cycles (median 10) of everolimus. Fifteen patients completed 12 cycles.
TABLE 1.
Patient Characteristics (n=23)
| Characteristic | Median (range) |
|---|---|
|
| |
| Age at enrollment (years) | 9.2 (3.8–17.1) |
|
| |
| Age at diagnosis (years) | 4.3 (0.3–11.8) |
|
| |
| Age at treatment (years) | 9.2 (3.8–17.1) |
|
| |
| Weight at enrollment (kg) | 28 (13–61) |
|
| |
| Body height (cm) | 129 (99–163) |
|
| |
| Body surface area (m2) | 1 (0.6–1.62) |
|
| |
| N (%) | |
|
| |
| Race | |
| White | 20/22 (91) |
| Black | 1/22 (5) |
| Other | 1/22 (5) |
| unknown | 1 |
|
| |
| Tumor location | |
| Brainstem | 1 (4) |
| Frontal lobe, Spinal Cord | 2 (9) |
| Hypothalamus | 2 (9) |
| Hypothalamus, basal ganglia | 1 (4) |
| Midbrain, thalamus | 1 (4) |
| Optic chiasm | 3 (13) |
| Optic chiasm, thalamus | 1 (4) |
| Optic nerve | 1 (4) |
| Optic pathway | 1 (4) |
| Posterior Fossa | 2 (9) |
| Right thalamus/brain stem | 1 (4) |
| Suprasellar | 2 (9) |
| Suprasellar cisternae | 1 (4) |
| Temporal lobe | 1 (4) |
| Temporal lobe, Cerebellar Peduncle, Posterior Cranial Fossa | 1 (4) |
| Thalamus | 2 (9) |
|
| |
| Number of prior chemotherapy regimens | |
| One | 2 (9) |
| Two | 8 (35) |
| More than two | 13 (56) |
|
| |
| Gender | |
| Male | 6 (26) |
| Female | 17 (74) |
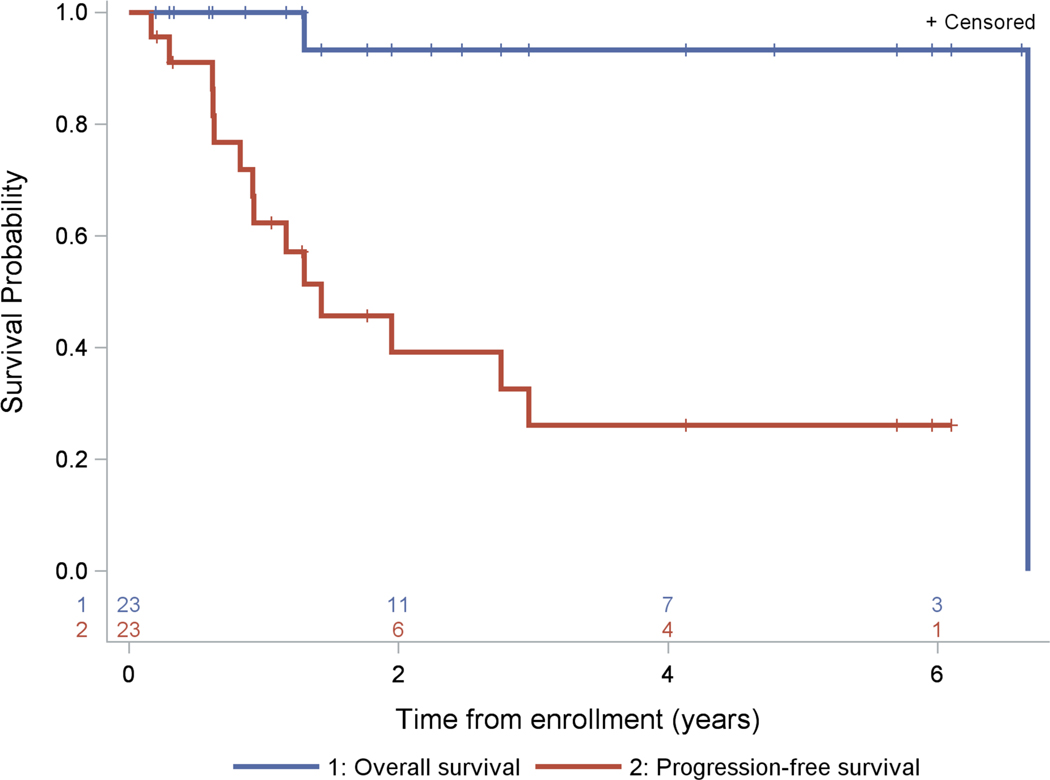
The response rate was 52.2% (12/23) with 2 PRs and 10 SD by end of cycle 12. Ten patients progressed prior to cycle 12despite SD at an earlier time in the trial. One additional patient with SD at week 17 was classified as a non-responder (PD) due to less than 48 weeks of follow-up (Table 2). For the 12 responders, the median time to best response was 0.9 months (range: 0.7–3.7 months). For the 10 patients reporting PD, progression occurred at a median of 0.95 months (range: 0.8–22.2 months). Ultimately, 14 patients developed progressive disease. Applying the one-stage rule, with10 responders of the first 20 evaluable patients, there is evidence to support continued study of everolimus. Assessment of response by the institutional radiologist agreed with the blinded central review performed by a single pediatric neuroradiologist.There were 14 events, including two patients who died. The 2, 3, and 5-yearprogression-free survival (PFS) were 39±11%, 26±11% and 26±11%, respectively. The 2, 3 and 5-year overall survival (OS) were each 93±6% (Fig. 1). The median follow-up time of patients without an event was 1.8 years (range: 0.2–6.7 years).
TABLE 2.
Number and proportion of patients, by response*, based on 2D radiographic imaging (n=23)
| Response | N (%) | Median [range] time to best response (months) | If CR/PR/SD, time to best response. If PD, time to PD.(months) |
|---|---|---|---|
| CR | 0 (0) | NA | NA |
| PR | 2 (13) | 2.3 (0.9–3.7) | 0.9, 3.7 |
| SD | 10 (48) | 0.9 (0.7–3.6) | 0.7, 0.8, 0.8, 0.9, 0.9, 0.9, 0.9, 0.9, 1.1, 3.6 |
| PD within 48 weeks** | 11 (39) | 0.95 (0.8–22.2)(n=10) | 0.8, 0.9, 0.9, 0.9, 0.9, 1.0, 2.5, 3.6, 7.3, 22.2 |
NA – not applicable
Patients who demonstrated progressive disease within the 48 weeks of protocol therapy were counted as non-responders.
One patient with best response of SD at week 17 but less than 48 weeks follow-up (went off study for toxicity) was classified as having PD (non-responder), because it was not possible to rule out progressive disease by week 48. The time to PD is unknown for this patient.
FIGURE 1.
Kaplan-Meier curves of progression-free survival and overall survival (n=23)
Overall, treatment was well tolerated by the majority of patients (see Supplementary Table S2). Seventeen patients had at least one grade 3 (n=15) or grade 4 (n=5) toxicity; six of these 17 experienced at least one grade 3 or 4 toxicityattributed to therapy. Grade 4 elevation in liver enzymes was deemed possibly related to everolimus; no grade 5 events occurred. There was one episode of grade 3 unrelated pneumonitis. Three grade 3 toxicities definitively attributed to everolimus therapyincludedmucositis and neutropenia in one patient and mucositis in another patient.
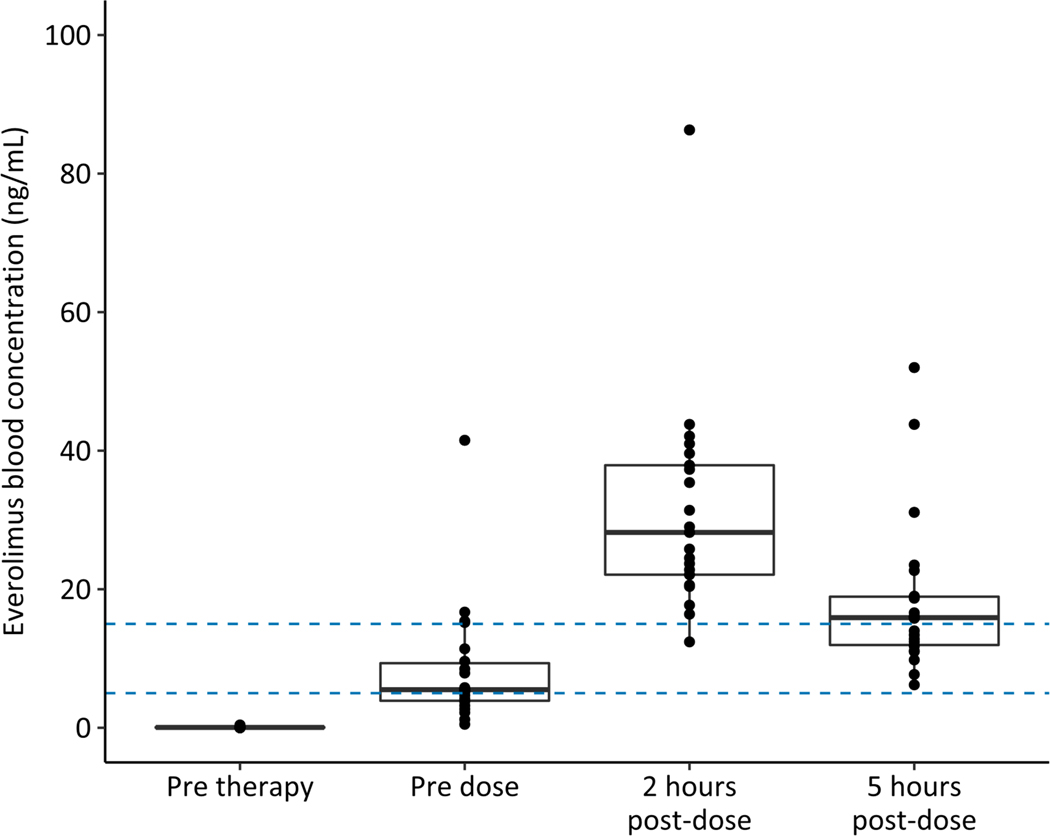
Pharmacokinetic data was available for 22 of 23 patients. Everolimus pharmacokinetic profilesexhibited substantial interpatient variability (Fig. 2 and Table 3). Comparably, the observed troughconcentrations in patients with TSwere below 5 ng/mL in 45.5% of patients with 18.2% of concentrations above 15 ng/mL.15Estimates of oral clearance and oral volume of distribution of the central compartment (Vc/F: 36.7 ± 20.2 L) were comparable with findings in the previous Phase I study of everolimus in pediatric patients with refractory solid tumors (Table 3).25
FIGURE 2.
Effective RAD001 (Everolimus) systemic exposure/pharmacokinetics (mean ± standard error of the mean) (n=22)
TABLE 3.
Everolimus pharmacokinetic parameter estimates
| Parameters | Mean | SD |
|---|---|---|
| Ctrough (ng/mL) | 7.9 | 8.8 |
| AUC0–24h (ng·h/mL) | 400 | 256 |
| CL/F (L/h/70 kg) | 29.6 | 14.0 |
| Vc/F (L/70 kg) | 84.1 | 30.3 |
| Q/F (L/h/70 kg) | 67.5 | 24.4 |
| Vp/F (L/70 kg) | 473 | 187 |
| Ka (h−1) | 2.00 | 0.61 |
Ctrough estimated trough blood concentration at 24 hours post-dose; AUC0–24h, area under the concentration-time curve for time 0 to 24 hours; CL/F, oral clearance; Vc/F, oral volume of distribution of the central compartment; Q/F, oral inter-compartmental clearance; Vp/F, oral volume of distribution of the peripheral compartment; Ka, absorption rate constant.
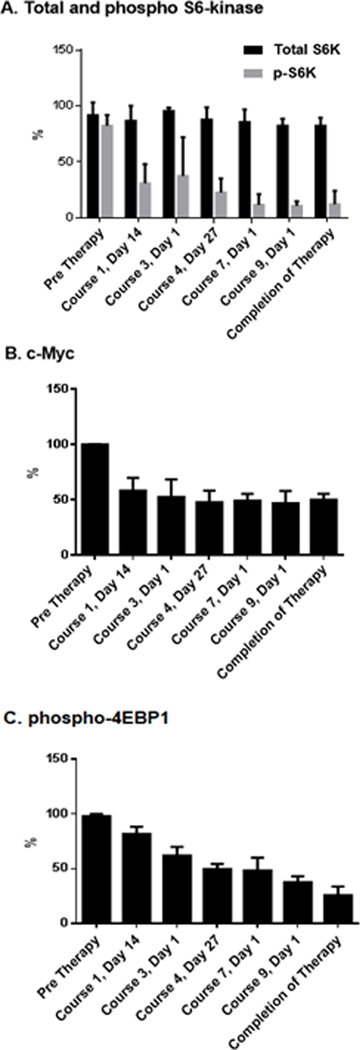
The pharmacodynamic activity of everolimus was assessed by analysis of inhibition of phosphorylation of S6, p70S6K, and eukaryotic translation initiation factor 4E binding protein 1 (p-4E-BP1), through IHC and expression of c-Myc (Fig. 3). Pharmacodynamic analysis of tumor samples obtained prior to enrollment on this study was performed in 8 cases (35%). Significantly decreased levels of phosphorylated 4E-BP1 and S6K were observed at the end of the first week of therapy; inhibition persisted through the duration of the study. While analysis of tumor mTOR inhibition, which would require biopsy of these brain tumors, could not be justified due to the risks involved in this patient population, the notable response rate suggests that the PK levels achieved were clinically relevant and similar to those observed in patients with TS responsive to everolimus.15
FIGURE 3.
Pharmacodynamic markers. (A) Sustained modulation of S6K phosphorylation and (B) c-MYC expression with Everolimus. (C) Progressive modulation of 4E-BP1 phosphorylation with Everolimus. Data was normalized to highest value within each patient group using two-way repeated measures ANOVA (n=8)
Discussion
Everolimus, a once daily oral mTOR inhibitor, demonstrated single agent activity in pediatric patients with radiographically progressive LGGs after standard chemotherapy and/or radiotherapy. After up to one year of treatment, 74% of patients maintained tumor growth arrest or shrinkage and showed no clinical progression. The therapy was easily administered as capsules or liquid, allowing for accurate dosing in all patients, did not require central venous access for administration, and was welltolerated in most patients.
Chemotherapy is standard treatment for pediatric patients with unresectable progressive LGGs.3 Variable response rates have been observed among differing drug regimens, yet the majority of patients do experience periods of tumor growth arrest interspersed with periods of progression requiring treatment. Despiteperiods of tumor progression, the majority of patients will be long-term survivors,2 reinforcing the importance of developing well-tolerated regimens without severe lifelong toxicities.6
Our initial understanding of pediatric LGGs was derived from two common genetic diseases, NF1 and TS,for which affected patients have a unique clinical course related to mutations in neurofibromin and TSC1/TSC2, respectively.31–34Sporadic pediatric LGGs rarely have mutations in these genes; rather, their mutations are found predominantly within BRAFin one of two common forms. Highly enriched in posterior fossa pilocytic astrocytomas, but identified in all pediatric LGG variants, is the truncated fusion event of BRAF, resulting in loss of its inhibitory domain with translocation to the KIAA1549 gene.35–37 A less frequent abnormality found across the different subtypes of pediatric LGGs is the BRAFV600E point mutation. Based on these data, pediatric LGG isconsidered a RAS/RAF/mTOR pathway disease,38 and consequently, mTOR inhibitors, having shown significant activity in TS patients (for which they are FDA approved), were suggested as treatment for those with sporadic progressive LGG.
mTOR is a downstream component of the PI3/AKT pathway and has two primary substrates, e1F-4E binding protein (4E-BP1) and p70 S6 kinase 1 (S6K1), which regulate translation of important messages, including those encoding the HIF-1 proteins, C-MYC, ornithine decarboxylase, cyclin D1, and the ribosomal proteins themselves. The drug is effective at nanomolar concentrations, and extensive pharmacologic testing in adults and children undergoing organ transplantation have demonstrated overall stable AUCs, supporting the use of standardized dosing. Based on body size, the pharmacology of everolimus is similar in adults and children,39 and efficacy in oncology trials is evident at clinically relevant doses.40,41Real-time pharmacokinetic analysis has been validated and the range of 5–15ng/ml is considered optimal.42 Specifically, pharmacokinetic-pharmacodynamic modeling based on inhibition of a peripheral molecular marker (S6 K1 activity in peripheral blood mononuclear cells) suggests that 5–10 mg daily in adults should be an adequate dose to produce a high degree of sustained target inhibition. Moreover, pharmacodynamic studies investigating changes in the molecular pathology of biopsied tumor by immunohistochemistry in treated patients at 5 and 10mg daily are associated with dephosphorylation of protein effectors known to be immediately downstream of mTOR such as S6 and 4EBP1. Pharmacodynamic analysis of tumor samples in our 8 pediatric cases at a dose of 5 mg/m2 was consistent with sustained modulation of S6K phosphorylation and c-Mycexpression and progressive modulation of 4EBP1. This near-total S6 and partial 4EBP1 inhibition observed in patients mirrors the results observed pre-clinically in in vivo models in which everolimus demonstrated clear anti-tumor activity.
It is difficult to compare response rates between LGG studies for a number of reasons. Generally speaking, tumorhistologies are heterogeneous. The spontaneous cessation of growth by LGGs over time also complicates the analysis of this patient population, however, we opted to evaluate stable disease within the response criteria unlike other studies. All patients were required to have evidence of radiographic progressionat time of enrollment to better measurethe drug’s true effect on tumor growth.The relationship to BRAF was not yet known at the time of this trial and therefore not incorporated into the analysis or outcomes, further complicating comparisons to more recent studies. Therefore, from a statistical point of view, we cannot make a valid comparison of outcomes between trials given the number of variables among the studies.
Certainly, clinicians may ask how these results fit into the treatment of LGG in the era of MAPK/MEK/BRAF inhibition. Taking into consideration the 5-year OS and PFS, this drug compares similarly to other standard chemotherapy trials for refractory disease utilizing vinblastine or upfront trials utilizing carboplatin/vincristine.6,9,43,44,45 It is difficult to make comparative statements regarding other targeted agents like selumetiniband trametinib, however,for whom the early data is descriptivein terms of sustained partial responses or includescombined cohorts of low grade patients treated with different BRAF/MEK inhibitors.46–48
In conclusion, everolimusdoes demonstrate activity in pediatric patients with radiographically progressive LGGs and provides another potentialoption for patients with recurrent disease. Itslimited,reversible toxicity and administrationas a pill or liquid preparationobviatesthe need for intravenous access. Furthermore, demonstration of synergy between everolimus and carboplatin in pediatric LGG cell lines and slowed tumor growth in in vivopediatric LGG models support its potential utility in future multi-agent protocols.49 Lastly, aseparate recentlycompleted phase II study of everolimusby the Pacific Neuro-Oncology Consortiumrequiring tissue at enrollment may provide further insight into its relevance for molecular subtypes of pediatric LGG while addressing quality of life measures and functional outcomes, which are now recognized as paramount to pediatric LGG assessments of response.
Supplementary Material
TABLE S1 Management of non-infectious pneumonitis
* A bronchoscopy with biopsy and/or bronchoalveolar lavage is recommended.
TABLE S2 Number of patients who experienced grade 3 or higher adverse events.
Within a given toxicity, each patient is counted only once at the highest grade. Seventeen of 23 patients had at least one grade ≥3 toxicity.
Acknowledgments:
The authors posthumously thank Dr. Robert Arceci, original study PI for Johns Hopkins Hospital, for his participation. This work was supported by the Pediatric Low-Grade Astrocytoma Foundation, Credit Unions Kids at Heart Team; Joe Andruzzi Foundation; Stop and Shop Pediatric Brain Tumor Fund, CJ Buckley Pediatric Brain Tumor Fund; Hamilton Team Chickeroo and the National Cancer Institute of the National Institutes of Health under award number P50CA165962. Administrative support for the trial was provided by the POETIC Consortium Operations Office. Drug was provided by Novartis Pharmaceutical.
Abbreviations
- ANC
absolute neutrophil count
- AUC
area under curve
- CNS
central nervous system
- CR
complete response
- CTCAE
common terminology criteria for adverse events
- CYP3A
cytochrome P450, family 3, subfamily A
- EDTA
ethylenediaminetetraacetic acid
- INR
international normalized ratio
- LDL
low-density lipoproteins
- LGG
low-grade glioma
- MRI
magnetic resonance imaging
- mTOR
mammalian target of rapamycin
- NF1
neurofibromatosis type 1
- OS
overall survival
- PBMC
peripheral blood mononuclear cells
- PD
pharmacodynamics
- PFS
progression-free survival
- PK
pharmacokinetics
- PR
partial response
- SD
stable disease
- TS
tuberous sclerosis
- ULNFA
upper limit of normal for age
Footnotes
Conflict of interest: M.W.K servedasa paid scientific advisory board member and received travel support from Novartis Pharmaceutical.
Kieran MW, Yao X, Macy M, Leary S, Cohen K, MacDonald T, Allen J, Boklan J, Smith A, Nazemi K, Gore L, Trippett T, DiRenzo J, Narendran A, Perentesis J, Prabhu S, Pinches N, Robison N, Manley P, Chi S. Final results of a prospective multi-institutional phase Ii study of everolimus (RAD001), an mTOR inhibitor, in pediatric patients with recurrent or progressive low-grade glioma.. A POETIC Consortium Trial. Neuro Oncol 2014;16(Suppl 3):iii27. 16th International Symposium on Pediatric Neuro-Oncology, Singapore.
Data Availability:
The data that support the findings of this study are available from the corresponding author upon reasonable request. Furthermore, results are available at www.clinicaltrials.gov, NCT00782626.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: IARC Press; 2007. 547p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandopadhayay P, Bergthold G, London WB, Goumnerova LC, Morales La Madrid A, Guo D, Ullrich NJ, Robison NJ, Chi SN, Beroukhim R, Kieran MW, Manley PE. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database.Pediatr Blood Cancer 2014;61:1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergthold G,Bandopadhayay P, Bi WL,Ramikisson L, Stiles C, Beroukhim R, Ligon KL, Grill J, Kieran MW. Pediatric low-grade gliomas: how modern biology reshapes the clinical field.BiochemBiophys Acta 2014;1845:294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Packer RJ, Lange B, Ater J, Nicholson HS, Allen J, Walker R, Prados M, Jakacki R, Reaman G, Needles MN. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol1993;11:850–856. [DOI] [PubMed] [Google Scholar]
- 5.Heath JA, Turner CD, Poussaint TY, Scott RM, Goumnerova L, Kieran MW. Chemotherapy for progressive low-grade gliomas in children older than ten years: the Dana-Farber experience.PediatrHematol Oncol 2003;20:497–504. [DOI] [PubMed] [Google Scholar]
- 6.Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, Lazarus KH, Packer RJ, Prados M, Sposto R, Vezina G, Wisoff JH, Pollack IF. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol 2012;30:2641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gururangan S, Fangusaro J, Poussaint TY, McLendon RE, Onar-Thomas A, Wu S, Packer RJ, Banerjee A, Gilbertson RJ, Fahey F, Vajapeyam S, Jakacki R, Gajjar A, Goldman S, Pollack IF, Friedman HS, Boyett JM, Fouladi M, Kun LE. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas--a Pediatric Brain Tumor Consortium study. Neuro Oncol 2014;16:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholson HS, Kretschmar CS, Krailo M, Bernstein M, Kadota R, Fort D, Friedman H, Harris MB, Tedeschi-Blok N, Mazewski C, Sato J, Reaman GH. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors - A report from the Children’s Oncology Group. Cancer 2007;110:1542–1550. [DOI] [PubMed] [Google Scholar]
- 9.Bouffet E, Jakacki R, Goldman S, Hargrave D, Hawkins C, Shroff M, Hukin J, Bartels U, Foreman N, Kellie S, Hilden J, Etzl M, Wilson B, Stephens D, Tabori U, Baruchel S. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol 2012;30:1358–1363. [DOI] [PubMed] [Google Scholar]
- 10.Robison NJ, Campigotto F, Chi SN, Manley PE, Turner CD, Zimmerman MA, Chordas CA, Werger AM, Allen JC, Goldman S, Rubin JB, Isakoff MS, Pan WJ, Khatib ZA, Comito MA, Bendel AE, Pietrantonio JB, Kondrat L, Hubbs SM, Neuberg DS, Kieran MW. A phase II trial of a multi-agent oral antiangiogenic (metronomic) regimen in children with recurrent or progressive cancer. Pediatric Blood and cancer 2014;61: 536–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercurio S, Padovani L, Colin C, Carre M, Tchoghandjian A, Scavarda D, Lambert S, Baeza-Kallee N, Fernandez C, Chappe C, Andre N, Figarella-Branger D. Evidence for new targets and synergistic effect of metronomic celecoxib/fluvastatin combination in pilocytic astrocytoma. Acta NeuropatholCommun 2013;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zapletalova D, André N, Deak L, Kyr M, Bajciova V, Mudry P, Dubska L, Demlova R, Pavelka Z, Zitterbart K, Skotakova J, Husek K, Martincekova A, Mazanek P, Kepak T, Doubek M, Kutnikova L, Valik D, Sterba J. Metronomic chemotherapy with the COMBAT regimen in advanced pediatric malignancies: a multicenter experience. Oncology 2012;82:249–60. [DOI] [PubMed] [Google Scholar]
- 13.Pachow D,Wick W,Gutmann DH, Mawrin C. The mTOR signaling pathway as a treatment target for intracranial neoplasms. Neuro Oncol 2015;17:189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth.Nature 2006;441:424–430. [DOI] [PubMed] [Google Scholar]
- 15.Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T, Franz DN. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med2010;363:1801–11. [DOI] [PubMed] [Google Scholar]
- 16.Franz DN, Belousova E, Sparagana, Bebin, Frost, Kuperman R, Witt O, Kohrman MH, Flamini JR, Wu JY, Curatolo P, de Vries PJ, Whittemore VH, Thiele EA, Ford JP, Shah G, Cauwel H, Lebwohl D, Sahmoud T, Jozwiak S. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2013; 381:125–132. [DOI] [PubMed] [Google Scholar]
- 17.Franz DN, Belouusova E, Sparagana S, Bebin EM, Frost M, Kuperman F, Witt O, Kohrman MH, Flamini JR, Wu JY, Curatolo P, de Vries PJ, Berkowitz N, Anak O, Niolat J, Jozwiak S. Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol 2014;15:1513–1520. [DOI] [PubMed] [Google Scholar]
- 18.Bar EE, Lin A, Tihan T, Burger PC, Eberhart CG. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol 2008; 67:878–887. [DOI] [PubMed] [Google Scholar]
- 19.Jones DT, Kocialkowski S, Liu L, Pearson DM, Backlund LM, Ichimura K, Collins VP. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas.Cancer Res 2008;68:8673–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfister S, Janzarik WG, Remk M, Ernst A, Werft W, Becker N, Toedt G, Wittmann A, Kratz C, Olbrich H, Ahmadi RB, Joos S, Radlwimmer B, Kulozik A, Pietsch T, Herold-Mende C, Gnekow A, Reifenberger G, Korshunov A, Scheurlen W, Omran H, Lichter P. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest 2008;118:1739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacConaill LE, Campbell CD, Kehoe SM, albas AJ, Hatton C, Niu L, Davis M, Yao K, Hanna M, Mondal C, Luongo L, Emery CM, Baker AC, Philips J, Goff DJ, Fiorentino M, Rubin MA, Polyak K, Chan, Wang, Fletcher JA, Santagata S, Corso, Roviello F, Shivdasani R, Kieran MW, Ligon KL, Stiles CD, Hahn WC, Meyerson ML, Garraway LA.Profiling critical cancer gene mutations in clinical tumor samples.PLoS One 2009; 18;4:e7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez FJ, Raabe EH. mTOR: a new therapeutic target for pediatric low-grade glioma? CNS Oncol 2014; 3:89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anglicheau D, Le Corre D, Lechaton S, Laurent-Puig P, Kreis H, Beaune P, Legendre C, Thervet E. Consequence of genetic polymorphisms for sirolimus requirements after renal transplant in patients on primary sirolimus therapy. Am J Transplant 2005; 5: 595–603. [DOI] [PubMed] [Google Scholar]
- 24.Mourad M, Mourad G, Wallemacq P, Garrigue V, Van Bellingen C, Van Kerckhove V, De Meyer M, Malaise J, Eddour DC, Lison D, Squifflet JP, Haufroid V. Sirolimus and tacrolimus trough concentrations and dose requirements after kidney transplantationin relation to CYP3A5 and MDR1 polymorphisms and steroids. Transplantation 2005; 80: 977–984. [DOI] [PubMed] [Google Scholar]
- 25.Fouladi M, Laningham F, Wu J, O’Shaughnessy MA, Molina K, Broniscer A, Spunt SL, MLuckett I, Stewart CF, Houghton PJ, Gilbertson RJ, Furman WL. Phase I study of everolimus in pediatric patients with refractory solid tumors.J Clin Oncol 2007; 25:4806–4812. [DOI] [PubMed] [Google Scholar]
- 26.Anderson BJ, Holford NH. Tips and traps analyzing pediatric PK data.PaediatrAnaesth 2011;21:222–37. [DOI] [PubMed] [Google Scholar]
- 27.Anderson BJ, Holford NH.Mechanism-based concepts of size and maturity in pharmacokinetics.Annu Rev PharmacolToxicol 2008;48:303–332. [DOI] [PubMed] [Google Scholar]
- 28.Weiss B, Widemann BC, Wolters P, Dombi E, Vinks AA, Cantor A, Korf B, Perentesis J, Gutmann DH, Schorry E, Packer R, Fisher MJ. Sirolimus for non-progressive NF1-associated plexiform neurofibromas: an NF clinical trials consortium phase II study. Pediatr Blood Cancer 2014;61:982–986. [DOI] [PubMed] [Google Scholar]
- 29.Weiss B, Widemann BC, Wolters P, Dombi E, Vinks A, Cantor A, Perentesis J, Schorry E, Ullrich N, Gutmann DH, Tonsgard J, Viskochil D, Korf B, Packer RJ, Fisher MJ. Sirolimus for progressive neurofibromatosis type 1-associated plexiform neurofibromas: a neurofibromatosis Clinical Trials Consortium phase II study. Neuro Oncol 2015;17:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hütt-Cabezas M, Karajannis MA, Zagzag D,Shah S, Horkayne-Szakaly I, Rushing EJ, Cameron JD, Jain D, Eberhart CG, Raabe EH, Rodriguez FJ. Activation of mTORC1/mTORC2 signaling in pediatric low-grade glioma and pilocytic astrocytoma reveals mTOR as a therapeutic target. Neuro Oncol 2013;15:1604–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutmann DH, Wood DL, Collins FS.Identification of the neurofibromatosis type 1 gene product. Proc Natl Acad Sci U S A 1991;88:9658–9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlmann EJ, Wong M, Baldwin RL,Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada K, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol 2002;52:285–296. [DOI] [PubMed] [Google Scholar]
- 33.Uhlmann EJ, Apicelli AJ, Baldwin RL, Burke Sp, Bajenaru ML, Onda H, Kwiatkowski D, Gutmann DH. Heterozygosity for the tuberous sclerosis complex (TSC) gene products results in increased astrocyte numbers and decreased p27-Kip1 expression in TSC2+/− cells. Oncogene 2002;21:4050–4059. [DOI] [PubMed] [Google Scholar]
- 34.Chan JA, Zhang H, Roberts PS, Jozwiak S, Wieslawa G, Lewin-Kowalik J, Kotulska K, Kwiatkowski DJ. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J Neuropathol Exp Neurol 2004;63:1236–1242. [DOI] [PubMed] [Google Scholar]
- 35.Raabe E, Kieran MW, Cohen KJ. New strategies in pediatric gliomas: molecular advances in pediatric low-grade gliomas as a model. Clin Cancer Res 2013; 19:4553–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergthold G, Bandopadhayay P, Hoshida Y, Ramkissoon S, Ramkissoon L, Rich B, Maire CL, Paolella BR, Schumacher SE, Tabak B, Ferrer-Luna R, Ozek M, Sav A, Santagata S, Wen PY, Gounerova LC, Ligon AH, Stiles C, Segal R, Golub T, Grill J, Ligon KL, Chan JA, Kieran MW, Beroukhim R. Expression profiles of 151 pediatric low-grade gliomas reveal molecular differences associated with location and histological subtype. Neuro Oncol 2015;17:1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones DT, Hutter B, Jäger N, Korshunov A, Kool M, Warnatz HJ, Zichner R, Lambert SR, Ryzhova M, Quang DA, Fontebasso AM, Stutz AM, Hutter S, Zuckermann M, Sturm D, Gronych J, Lasitschka B, Schmidt S, Seker-Cin H, Witt H, Sultan M, Ralser M, Northcott PA, Hovestadt V, Bender S, Pfaff E, Stark S, Faury D, Schwartzentruber J, Majewski J, Weber UD, Zapatka M, Raeder B, Schlesner M, Worth CL, Bartholomae CC, von Kalle C, Imbusch CD, Radomski S, Lawerenz C, van Sluis P, Koster J, Volckmann R, Versteeg R, Lehrach H, Monoranu C, Winkler B, Unterberg A, Herold-Mende C, Milde T, Kulozik AE, Ebinger M, Schuhmann MU, Cho YJ, Pomeroy SL, von Deimling A, Witt O, Taylor MD, Wolf ML, Brors B, Felsberg J, Reifenberger G, Collins VP, Jabado N, Elis R, Lichter P, Pfister SM. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet 2013;45:927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendoza MC, Er EE, Blenis J.The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 2011;36:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovarik JM, Noe A, Berthier S, McMahon L, Langholff WK, Marion AS, Hoyer PF, Ettenger R, Rordorf C. Clinical development of an everolimus pediatric formulation: relative bioavailability, food effect, and steady-state pharmacokinetics. J Clin Pharmacol 2003;43:141–147. [DOI] [PubMed] [Google Scholar]
- 40.Ravaud A, Urva SR, Grosch K, Cheung WK, Anak O, Sellami DB.Relationship between everolimus exposure and safety and efficacy: meta-analysis of clinical trials in oncology. Eur J Cancer 2014;50:486–95. [DOI] [PubMed] [Google Scholar]
- 41.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, Martinelli E, Ramon y Cajal S, Jones S, Vidal L, Shand N, Macarulla T, Ramos FJ, Dimitrijevic S, Zoellner U, Tang P, Stumm M, Lane HA, Lebwohl D, Baselga J. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 2008;26:1603–1610. [DOI] [PubMed] [Google Scholar]
- 42.McMahon LM, Luo S, Hayes M, Tse FL.High-throughput analysis of everolimus (RAD001) and cyclosporin A (CsA) in whole blood by liquid chromatography/mass spectrometry using a semi-automated 96-well solid-phase extraction system. Rapid Commun Mass Spectrom 2000;14:1965–1971. [DOI] [PubMed] [Google Scholar]
- 43.Ater JL, Xia C, Mazewski C, Booth TN, Freyer DR, Packer RJ, Sposto R, Vezina G, Pollack. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: A report from the Children’s Oncology Group. Cancer 2016;15:1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouffet E, Jakacki R, Goldman S, Hargrave D, Hawkins C, Shroff M, Hukin J, Bartels U, Foreman N, Kellie S, Hilden J, Etzl M, Wilson B, Stephens D, Tabori U, Baruchel S. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol 2012; 30:1358–1363. [DOI] [PubMed] [Google Scholar]
- 45.Lassaletta A, Scheinemann K, Zelcer SM, Hukin J, Wilson BA, Jabado N, Carret AS, Lafay-Cousin L, Larouche V, Hawkins CE, Pond GR, Poskitt K, Keene D, Johnston D, Eisenstat DD, Krishnatry R, Mistry M, Arnoldo A, Ramaswamy V, Huang A, Bartels U, Tabori U, Bouffet E. Phase II weekly vinblastine for chemotherapy-naïve children with progressive low-grade glioma: a Canadian Pediatric Brain Tumor Consortium Study. J Clin Oncol 2016;34:3537–3543. [DOI] [PubMed] [Google Scholar]
- 46.Fangusaro J, Onar-Thomas A, Young Poussaint T, Wu S, Ligon AH, Lindeman N, Banerjee A, Packer RJ, Kilburn LB, Goldman S, Pollack IE, Qaddoumi I, Jakacki R, Fisher PG, Dhall G, Baxter P, Kreissman SG, Stewart CF, Jones DTW, Pfister SM, Vezina G, Stern JS, Panigrahy A, Patay Z, Tamrazi B, Jones JY, Haque SS, Enterline DS, Cha S, Fisher MJ, Doyle LA, Smith M, Dunkel IJ, Fouladi M. Selumetinib in paediatricpateints with BRAF-aberrant or neurofibromatosis type 1- associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol 2019; 20: 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nobre L, Zaptocky M, Ramaswamy V, Ryall S, Bennett J, Alderete D, Balaguer Guill J, Baroni L, Bartels U, Bavie A, Bornhorst M, Boue DR, Canete A, Chintagumpala M, Coven Sl, Cruz O, Dahiya S, Dirks P, Dunkel IJ, Eisenstat D, Faure Conter C, Finch E, Finlay JL, Frappsaz D, Garre ML, Gauvain K, Bechensteen AG, Hansford JR, Harting I, Hauser P, Hazrat L, Huang A, Injac SG, Lurilli V, Karajannis M, Kaur G, Kyncl M, Krskova L, Laperriere N, Larouche V, Lassaletta A, Leary S, Lin F, Mascelli S, McKeown T, Milde T, Quiroga-Cantero E, Rutka J, Sabel M, Salgado D, Solan P, Sterba J, Su J, Sumerauer D, Taylor MD, Toledano H, Tsang DS, Valente Fernandes M, van Landeghem F, vanTilbur CM, Wilson B, Witt O, Zamecnik J, Bouffet E, Hawkins C, Tabori U. Outcomes of BRAFV600E pediatric gliomas treated with targeted BRAF inhibition. JCO Precis Oncol 2020. (eCollection). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kondyli M, Larouche V, Saint-Martin C, Ellezam B, Pouliot L, Legault G, Crevuer Km Weil A, Farmer J, Jabado N, Perreault S. Trametinib for progressive pediatric low-grade gliomas. J Neurooncol. 2018: 140: 435–444. [DOI] [PubMed] [Google Scholar]
- 49.Poore B, Yuan M, Arnold A, Price A, Alt J, Rubens JA, Slusher BS, Eberhart CG, Raabe EH. Inhibition of mTORC1 in pediatric low-grade glioma depletes glutathione and therapeutically synergizes with carboplatin. Neuro Oncol 2019;21:252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Management of non-infectious pneumonitis
* A bronchoscopy with biopsy and/or bronchoalveolar lavage is recommended.
TABLE S2 Number of patients who experienced grade 3 or higher adverse events.
Within a given toxicity, each patient is counted only once at the highest grade. Seventeen of 23 patients had at least one grade ≥3 toxicity.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Furthermore, results are available at www.clinicaltrials.gov, NCT00782626.