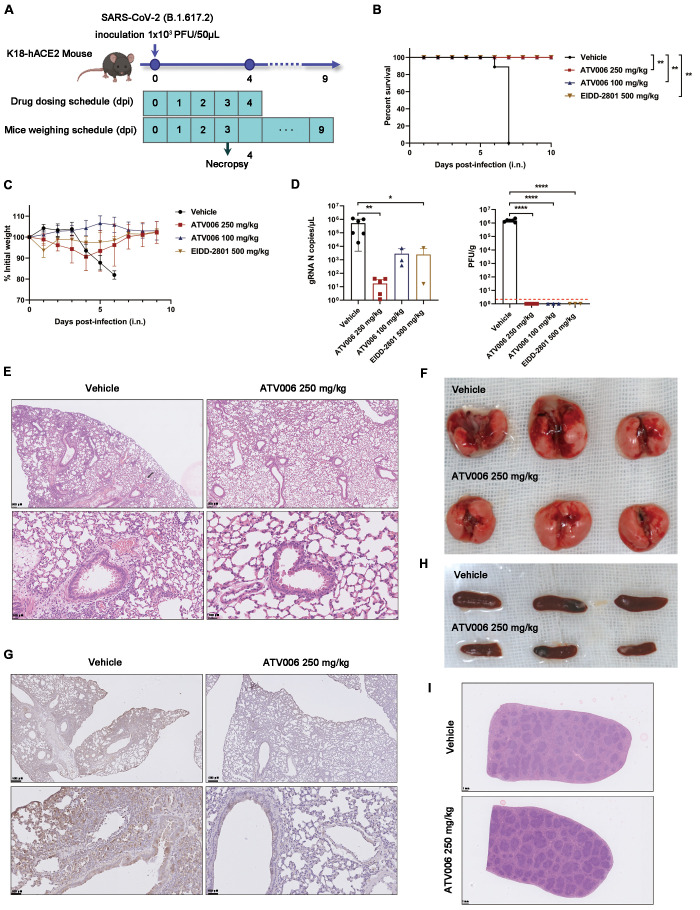
Fig. 5. Prophylactic ATV006 treatment is efficacious against SARS-CoV-2 in K18-hACE2 mice.
(A) The experimental timeline is shown. K18-hACE2 mice were intranasally inoculated with the SARS-CoV-2 Delta variant (1 × 103 PFU per mouse) and treated with vehicle (control, n=11), ATV006 (250 mg/kg, orally, once daily, n=11), ATV006 (100 mg/kg, orally, once daily, n=8) or EIDD-2801 (500 mg/kg, orally, once daily, n=8). (B) The survival curve is shown. (C) Change in body weight was measured over time post infection. (D) Viral load from lung tissue at 3 dpi was analyzed by qRT-PCR and plaque assay. The detection limit of qRT-PCR was 0.5 copies/μL. The red dashed line in (D, right panel) indicates the limit of detection for the plaque assay. (E to G) Histopathology (E), gross pathology (F), and immunohistochemistry detection of SARS-CoV-2 S protein (G) are shown for lungs isolated from the indicated treatment groups. S protein is denoted by brown staining in (G). (H and I) Gross pathology (H) and histopathology (I) are shown for spleens isolated from the indicated treatment groups. Data in (C and D) are presented as mean ± SD. Statistical analysis was conducted using a Log-Rank test (survival), a one-way ANOVA with Dunnett’s correction for multiple comparisons (lung titer), or a Kruskal-Wallis test with Dunn's correction for multiple comparisons (lung viral RNA). ∗p ≤ 0.05; ∗∗p ≤ 0.005; ∗∗∗∗p ≤ 0.0001.