Abstract
Objective:
The purpose was to evaluate whether patient and spouse cancer-specific distress mediated the association between cancer severity and occupational functioning among employed spouses of women diagnosed with breast cancer. We examined whether sociodemographic characteristics, lower spouse-reported marital quality, and lower spouse self-rated health were associated with poorer spouse occupational functioning.
Methods:
One hundred forty-three currently employed spouses of women diagnosed with breast cancer were administered measures of socioeconomic status, occupational functioning (work absenteeism, low productivity, and poor performance), cancer-specific distress, marital quality, and self-rated health. Patients completed measures of cancer-related distress and functional impairment and cancer stage were collected from medical charts.
Results:
In the model evaluating work absenteeism, greater patient functional impairment was associated with more absenteeism, but there was no evidence of a mediating effect for either partners’ cancer-specific distress. Higher cancer stage and more functional impairment were associated with higher spouse cancer-specific distress, which in turn predicted poorer work productivity. Patient cancer-specific distress did not mediate the association between patient functional impairment or cancer stage and spouse work productivity. Finally, higher cancer stage was associated with more spouse cancer-specific distress, which in turn predicted poorer work performance. There were no direct or indirect effects of the patient’s functional impairment on spouse work performance.
Conclusions:
Distressed spouses are more likely to have poorer work productivity after their partners’ breast cancer diagnosis. These spouses may need assistance in managing their distress and the patient’s functional impairment to ensure that their work productivity is not adversely affected.
Introduction
Given the decline in health care resources, reduction in inpatient hospitalizations, and the advent of home care, family caregivers, particularly spouses, have increasingly assumed a central role in cancer patients’ healthcare [1]. Family members assume a lion’s share of the practical and emotional assistance to patients. Family members are called upon to accompany patients to medical appointments, assist patients with home responsibilities such as housework, provide medical care at home, coordinate medical care, provide financial support, and frequently provide emotional support [2–4]. Providing practical and emotional support can come at a significant cost to caregivers in terms of altered daily routines, which compromised physical health, financial stress, and occupational issues [5]. Indeed, these stressors contribute to emotional distress among family members: Although most research suggests that overall partner distress levels are not significantly higher than levels in the general population [6], partners and other cancer caregivers express relatively high levels of cancer-related concerns and needs for support [7,8].
Among the adverse effects of being a spouse of a cancer patient, the occupational impact of the diagnosis of cancer on the spouse has received the least attention. There is an extensive literature on the occupational impact among cancer patients (see [9] for a review of this topic). Among women diagnosed with breast cancer, adverse occupational effects include reduced work hours [10–13], delayed return to work [14,15], losing one’s position [16,17], and a downgrading of one’s work status (e.g., lower paying position) [10].
Less attention has been paid to similar outcomes in terms of occupational impact of a cancer diagnosis on spouses, particularly among spouses of breast cancer patients. Northouse et al. [18] and Zahlis and Shands [19] used qualitative interviews to identify changes in occupational functioning among spouses of early stage breast cancer patients and reported that spouses reduced their work schedules to accommodate their wives’ care. Spouses also reported less productivity while at work: ‘I go to work and think about her 8 hours a day, come home’. Hollenbeack et al. [20] studied employment status in a sample of spouses of cancer patients who were between 2 and 6 years posttreatment. The sample consisted primarily of patients with stage 1 to 3 cancers (92%). Compared with a healthy control group, wives were significantly less likely to be employed. Van Houtven et al. [21] quantified the total economic burden of providing care to lung cancer and colorectal cancer patients. A subsample of caregivers in this study was spouses (64%), and 84% of these caregivers were providing care to patients with stages 1 to 3 cancers. There were significant costs associated with lost work, vacation, and sick time associated with providing care to the patient. Mosher et al. [22] evaluated distressed caregivers (62% spouses) of lung cancer patients. About 43% of the sample was diagnosed with stages 1 to 3 cancers. Approximately half of the caregivers reported that they worked fewer hours each week since the patient’s diagnosis, 18% reported quitting work to take care of the patient, and 28% of caregivers reported that a major source of family income was lost. In sum, the limited research on occupational impact on spouses of patients diagnosed with nonterminal stage cancers suggests that cancer may have adverse effects on their occupational functioning.
There is little known about factors contributing to the occupational impact of a cancer diagnosis on spouses. One factor that has been associated with spouse occupational functioning is the severity of the patient’s cancer, which has been defined as the level of functional impairment, symptom severity, and the cancer’s stage. Among spouses of patients with various types of cancer, a greater time since diagnosis [23] and a greater number of limitations in the ability of the cancer patient to perform activities of daily living [23] have been associated with lost work time among spouses. A second factor that has been associated with occupational functioning is the caregivers’ psychological state. Indeed, higher levels of caregiver distress have been associated with a greater loss of family income [22,24]. Sherwood et al. [23] found that depressed spouses providing care to cancer patients were less likely to be employed. A third factor that has been associated with spouse occupational functioning is sociodemographic characteristics. There is inconsistent data regarding age, with some studies suggesting that older caregivers report poorer occupational functioning [25] and some studies suggesting that younger caregivers report poorer occupational functioning [26]. Caregivers with lower incomes [23] and caregivers with more health problems [23] report poorer occupational functioning than caregivers with fewer health problems.
There are additional factors which have not been investigated that may play a role in spouse occupational functioning. One possible factor is the patient’s psychological functioning. Given the interdependent nature of the marital relationship and the strong correlation between cancer patient and spouse distress [27], it is likely that both patient and spouse cancer-related distress adversely affect spousal occupational functioning. A second possible factor is the quality of the marital relationship. Spouses who are in lower quality relationships are more likely to report depressive symptoms [28], and distress has been linked with poorer occupational functioning in studies in the general population (e.g., [29]). Thus, lower relationship quality may impact spouse occupational functioning either indirectly by engendering spouse distress or by directly by impacting spouse occupational functioning.
In the present study, we proposed a model of associations between patient disease severity, spouse demographic (age, education, and income) and health (medical conditions, self-rated health status, and health care utilization) factors, spouse and patient cancer-related distress, spouse marital quality, and spouse occupational functioning. Occupational functioning was defined in three ways: work absenteeism, which was defined as hours lost from work; low productivity while at work; and poor work performance. Based on prior research, we proposed a model (Figure 1) whereby the association between patient cancer severity (e.g., later stage cancer and more functional impairment) and spouse occupational functioning (absenteeism, low productivity, and poor performance) was mediated by both patient and spouse cancer-related distress. That is, it was proposed that greater patient cancer disease severity influenced occupational functioning because both patient and spouses experience greater distress when the breast cancer’s physical impact is higher. We also evaluated the role of spouse-reported marital quality and spouse self-rated health. We predicted that poorer marital quality and poorer spouse health would contribute to poorer occupational functioning indirectly by their association with spouse distress and by a direct association with occupational functioning.
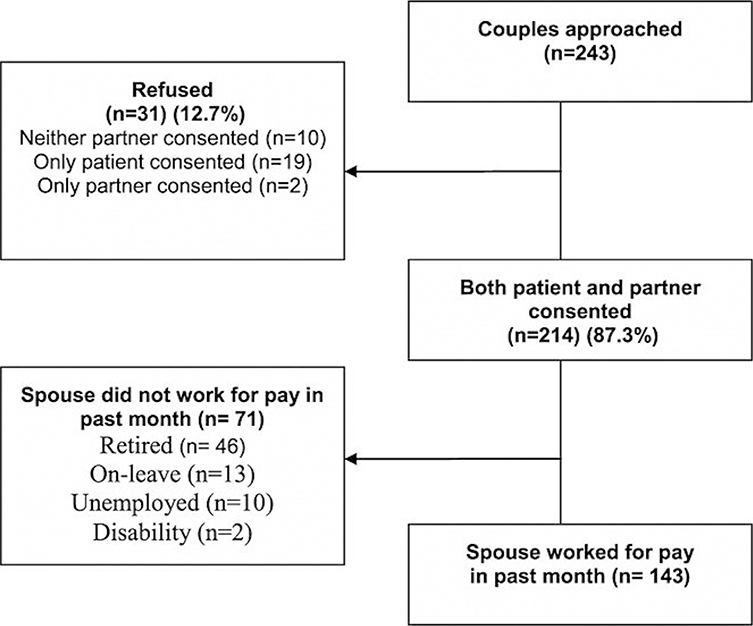
Figure 1.
CONSORT flowchart
Methods
Participants
Participants were 143 women with early stage breast cancer and their spouses drawn from the baseline data from a larger, ongoing randomized clinical trial evaluating the efficacy of a couple-focused group intervention for couples coping with breast cancer (Manne, unpublished). For clarity of presentation, the term spouse was used to denote the patient’s partner, even though there are some partners in the study who were not married to the patient.
Procedure
Patients were approached for study participation from the outpatient clinics of oncologists practicing in four cancer centers in the Northeastern USA or in several smaller local community hospital oncology practices. As noted earlier, participants were part of a longitudinal study of the efficacy of two eight-session couple-focused group interventions. Criteria for the current study inclusion were as follows: (a) patient had a primary diagnosis of ductal carcinoma in situ or stage 1, 2, or 3a breast cancer, (b) patient was female, (c) patient had breast cancer surgery, (d) patient and spouse were 18 years of age or older, (e) patient and spouse were able to give informed consent, (f) patient and spouse were English-speaking, (g) patient currently married or living with a significant other of either sex, and (h) spouse worked for pay in the past month.
Eligible patients were identified and approached either after an outpatient visit, by telephone contact, or by mail. Patient and spouse were given a written informed consent document and the main study’s baseline survey to complete and return by mail. Measures for the present study, named the work performance substudy (work performance, health care utilization, and medical conditions), were sent with the main study survey packet but were labeled ‘optional questionnaire’ with a separate consent form and reimbursement. Participants were provided $15 to complete the main study survey and $15 to complete the work performance substudy survey. The work performance substudy began in November, 2009, which was 2 years after the main study began. One hundred forty-three couples completed the substudy. The CONSORT diagram is shown in Figure 2. Comparisons were made between the 143 participants and the 31 substudy refusers with regard to available data (spouse age, spouse race/ethnicity, patient cancer stage, time since patient’s diagnosis, patient’s performance status, spouse and patient cancer-specific distress, and spouse relationship satisfaction). There were no statistically differences between the two groups.
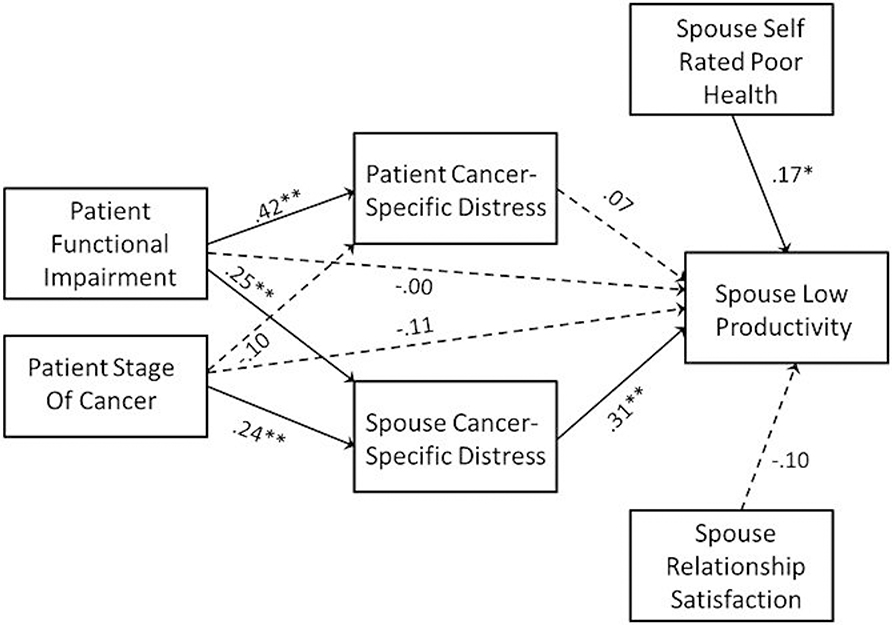
Figure 2.
Mediational model predicting spouse work productivity. Path coefficients are standardized, and dashed paths indicate path coefficients that were not significantly different from zero. Higher scores on the work productivity scale indicate poorer performance. *p < .05, **p < .01
Measures
Demographic variables
Spouses and patients reported age, income, ethnicity, and education.
Spouse occupational functioning: health and work performance questionnaire—clinical trials version [30]
The health and work performance questionnaire (HPQ) is a 13-item self-report instrument designed to estimate the workplace costs of health problems in terms of reduced job performance, sickness absence, and work-related accidents and injury. Although not used in cancer-related occupational functioning studies, this instrument was selected for two reasons: First, the HPQ has excellent psychometrics and is widely used. The measure is associated with archival measures of work loss and performance [30] and is widely used in studies evaluating work performance [31] and studies of the impact of health issues on work [32]. Second, the measure is one of the few instruments that includes an assessment of presenteeism, which measures being present, but not productive, at work. Presenteeism is a relatively new but important component of occupational functioning, particularly among spouses who may need to not lose work days in order to retain employment and a family income when their partner is ill with cancer. For the present study, we used the work situation items (employment status) and the absenteeism and presenteeism items. Although there are a number of different ways of scoring this instrument, the three scales we selected are the HPQ scales that have been validated and have correlations with independent measures of workplace performance [29,30,33]. We did not use items assessing type of work (professional and sales), the number of supervisees, how many hours the spouse was expected to work, or work achievements or failures, as they are not used in the three scales selected for analyses.
The absenteeism measure consisted of four items assessing the number of days of work missed because of problems with physical or mental health or other reasons. Two indicators of presenteeism were calculated. The first indicator consisted of seven items assessing low work productivity. Sample items were ‘How often was your work performance lower than most workers on your job’, and ‘How often was the quality of your work lower than it should have been?’ Higher scores indicated lower work productivity. For ease of interpretation, this variable was named ‘low work productivity’. The Cronbach’s alpha for the low work productivity scale was .72. The second indicator was a measure of self-reported poor work performance. The self-reported rating of overall poor performance on the days the person worked during the past 4 weeks ranged from 10 = worst job performance to 0 = performance of a top worker.
Spouse relationship satisfaction
Spouses completed the Dyadic Adjustment Scale [34]. The Dyadic Adjustment Scale is a widely used measure of satisfaction with intimate relationships. The scale is composed of 32 items assessing relationship consensus, satisfaction, cohesion, and affectional expression. Higher scores indicate more satisfaction. The maximum score is 151, and the cutoff score that was used to indicate marital distress was 97. Internal consistency in the present study, as calculated by Cronbach’s alpha, was .94.
Spouse health
Medical conditions:
Spouses reported whether they had been diagnosed with 12 health conditions: allergies, arthritis, asthma, diabetes, glaucoma, heart disease, high cholesterol, hypertension, lupus, migraines, anemia, and/or sleep apnea. A total score was created by summing the number of conditions.
Health care utilization:
Spouses reported how often they visited different types of physicians in the past year (e.g., internist, cardiologist, urologist, radiologist, Ear, Nose, and Throat (ENT), surgeon, oncologist, and neurologist). The score was the total number of visits in the past year.
Self-reported health status:
Spouses rated their overall health status using a single-item rating taken from the Medical Outcomes Scale, SF-12 [35]. This item asks participants to rate their health, in general, from ‘poor’ to ‘excellent’ using five descriptors (1 = ‘excellent’, 2 = ‘very good’, 3 = ‘good’, 4 = ‘fair’, and 5 = ‘poor’).
Spouse and patient cancer-specific distress
Participants completed the Impact of Events Scale [36], which is a 21-item self-report measure focusing on intrusive ideation and avoidance associated with a stressor, in this case breast cancer and its treatment. A sample item is, ‘I thought about it when I didn’t mean to’. This scale has been widely used as a measure of cancer distress (e.g., [37,38]). Using a 4-point Likert scale (0 = not at all, 1 = rarely, 3 = sometimes, and 5 = often), participants rated how true each statement was for them during the past week. Scale scores could range from 0 to 105. Internal consistency for the spouse scale, as calculated by Cronbach’s alpha, was .92, and the internal consistency for the patient scale was .90.
Patient functional impairment
The functional status subscale of the Cancer Rehabilitation Evaluation System [39] was used. Twenty-six items assessed functional disability caused by the cancer and its treatment. Participants rated difficulty during the past month from 0 (not at all) to 4 (very much). An example is ‘I find I have difficulty doing household chores’. Higher scores indicate higher levels of functional impairment. Internal consistency, as calculated by Cronbach’s alpha, was .93.
Patient medical status
Data regarding the patient’s disease stage (1 to 3a), treatment status (chemotherapy and radiation), time since diagnosis, and Eastern Cooperative Oncology Group symptom ratings (e.g., ‘0’ = no symptoms, fully active and able to work; ‘1’ = symptomatic, but not spending extra time in bed and able to do light work) were obtained from the medical chart.
Results
Descriptive information
Descriptive information regarding the study sample is shown in Table 1. Spouses tended to be relatively well-educated, white, in their mid-50s, and married more than 20 years. Patients were similarly well-educated, white, and in their mid-50s. In terms of medical status, over half of the patients had stage 1 or 2 cancers and underwent breast-conserving surgery. Spouses rated their health, on average, in the range of ‘very good’, reported an average of three physician visits in the past year (M = 3.3, range = 0–23), and the number of health conditions averaged about 1.6 (range = 0–8). Marital satisfaction averaged 115, which is higher than average (range = 63–149).
Table 1.
Sample characteristics (N = 143)
| Variable | Partners |
Patients |
||
|---|---|---|---|---|
| n (%) | M (SD) | n (%) | M (SD) | |
|
| ||||
| Gender | ||||
| Male | 143 (100) | 0 | ||
| Female | 0 | 143 (100) | ||
| Age (years) Ethnicity | 56.2 (8.9) | 52.3 (8.7) | ||
| Ethnicity | ||||
| White | 117 (81.8) | 124 (86.7) | ||
| Non-white | 22 (15.4) | 18 (12.6) | ||
| Missing | 4 (2.7) | 1 (.7) | ||
| Education | ||||
| <High School | 23 (16.1) | 22 (15.3) | ||
| Some College–College degree | 62 (43.4) | 70 (49.0) | ||
| Graduate work or degree | 56 (39.2) | 50 (35.0) | ||
| Missing | 2(1.3) | 1 (.6) | ||
| Median family income ($) | $100,000 | $100,000 | ||
| Marital status | ||||
| Married | 137 (95.6) | 137 (95.6) | ||
| Cohabitating | 6 (4.4) | 6 (4.4) | ||
| Relationship length (years) | ||||
| Married | 22.4 (12.0) | 22.4 (11.9) | ||
| Cohabitating | 9.3 (5.7) | 10.3 (5.3) | ||
| Stage of disease | ||||
| 0 | 29 (20.3) | |||
| 1 | 63 (44.1) | |||
| 2 | 38 (26.6) | |||
| 3a | 10 (6.9) | |||
| Missing | 3 (2.1) | |||
| ECOG rating | ||||
| 0 | 113 (79) | |||
| 1 | 16 (11.2) | |||
| Type of surgery | ||||
| Mastectomy | 54 (37.8) | |||
| Breast-conserving surgery | 89 (62.2) | |||
In terms of work absenteeism, approximately half of the sample missed less than three work days in the past month. On average, work productivity was relatively high (i.e., low work productivity was low), with approximately half of the sample reporting an average rating between ‘none of the time’ and ‘a little of the time’ with regard to difficulties concentrating at work, producing lower quality work than expected, and/or doing no work when the spouse was supposed to be working. Poor work performance was also relatively rare, with M = 2.06, SD = 1.43, and range between 0 and 6. Thus, levels of work performance were relatively high.
Correlations, means, and standard deviations for the variables included in the models are presented in Table 2. Higher spouse absenteeism was significantly correlated with higher levels of cancer-specific distress both on the part of the patient and spouse, as well as higher levels of patient functional impairment. Lower work productivity was associated with greater cancer-specific distress on the part of patients and spouses, as well as lower spousal relationship satisfaction and poorer spouse self-rated health. Poor work performance was significantly correlated with lower spouse relationship satisfaction and poorer spouse self-rated health.
Table 2.
Descriptive statistics and correlations on variables examined for inclusion in the mediational models
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 1. Sp low work prod | |||||||||||
| 2. Sp absenteeism | 29** | ||||||||||
| 3. Sp poor work perf | .64** | .22** | |||||||||
| 4. Pt funct impairment | .11 | .21* | .02 | ||||||||
| 5. Pt cancer stage | −.02 | −.04 | .04 | .17* | |||||||
| 6. Sp cancer distress | .35** | .08 | .24** | .29** | .28** | ||||||
| 7. Pt cancer distress | .18* | .17* | −.02 | .40** | −.04 | 31** | |||||
| 8. Sp relationship sat | .24** | −.18* | −.21* | −.15 | −.03 | .24** | −.26** | ||||
| 9. Sp poor SR health | .24** | .11 | .25** | −.02 | .02 | .14 | −.06 | −.35** | |||
| 10. Sp health care use | .06 | .03 | .11 | −.12 | .01 | −.07 | −.23* | −.02 | .18* | ||
| 11. Sp med conditions | .07 | −.10 | .04 | −.16 | −.05 | −.10 | −.20* | −.07 | .32** | .37** | |
| M | 12.63 | 3.45 | 2.06 | 19.35 | 1.34 | 23.41 | 33.58 | 116.28 | 2.26 | 3.3 | 1.6 |
| SD | 3.47 | 3.94 | 1.43 | 16.60 | 1.13 | 18.29 | 20.93 | 16.66 | .79 | 3.7 | 1.5 |
Pt, patient; Sp, spouse; Perf, performance; Prod, productivity; Funct, functional; Sat, satisfaction; SR, self-rated; Med, medical.
p < .05.
p < .01.
Mediational models predicting spouse occupational functioning
Analytic approach
The mediational model proposed that the patient’s medical status, specifically cancer stage and functional impairment, would be associated with the spouse’s work absenteeism, low work productivity, and poor work performance, and that these associations would be mediated by the spouse’s cancer-specific distress and the patient’s cancer-specific distress. Mediational analyses were conducted using AMOS with bootstrapping to compute 95% confidence intervals for the indirect effects. Prior to estimating the model, we explored a series of possible covariates to be included in the model by examining the zero-order associations between the covariates and spouse low work productivity, absenteeism, and poor work performance. Variables considered as possible covariates included the following variables for the spouse: age, education, income, self-rated poor health, number of health conditions, health limitations to activity, and relationship satisfaction. We also considered as possible covariates the partner’s relationship satisfaction, the number of comorbid health conditions, and Eastern Cooperative Oncology Group (ECOG) rating. Of all these, only the spouse’s self-rated poor health and spouse’s relationship satisfaction correlated significantly with absenteeism, low work productivity, and poor work performance. Thus, these two variables were included as covariates in the mediational models.
Model predicting spouse work productivity
As can be seen in Figure 1, although there is no evidence of direct effects of the patient’s medical status on the spouse’s productivity, there is evidence that the patient’s cancer stage and functional impairment both positively predict the spouse’s cancer-specific distress, which in turn predicts poorer work productivity. The indirect effect of stage on work productivity via the spouse’s distress was b = .221; 95% CI = .013–.453, β = .069; 95% CI = .003–.146, p = .034. Likewise, there was evidence of an indirect effect of patient functional impairment on spouse work productivity via spouse cancer-specific distress with the indirect effect of b = .022; 95% CI = .004–.045, β = .107; 95% CI = .017–.211, p = .017. In contrast, there was no evidence that the effects of patient functional impairment or cancer stage on the spouse’s work productivity were mediated by the patient’s cancer-specific distress. In sum, spouse low work productivity was associated with the patient’s medical status to the extent that the spouse experienced higher cancer-specific distress.
Model predicting spouse work absenteeism
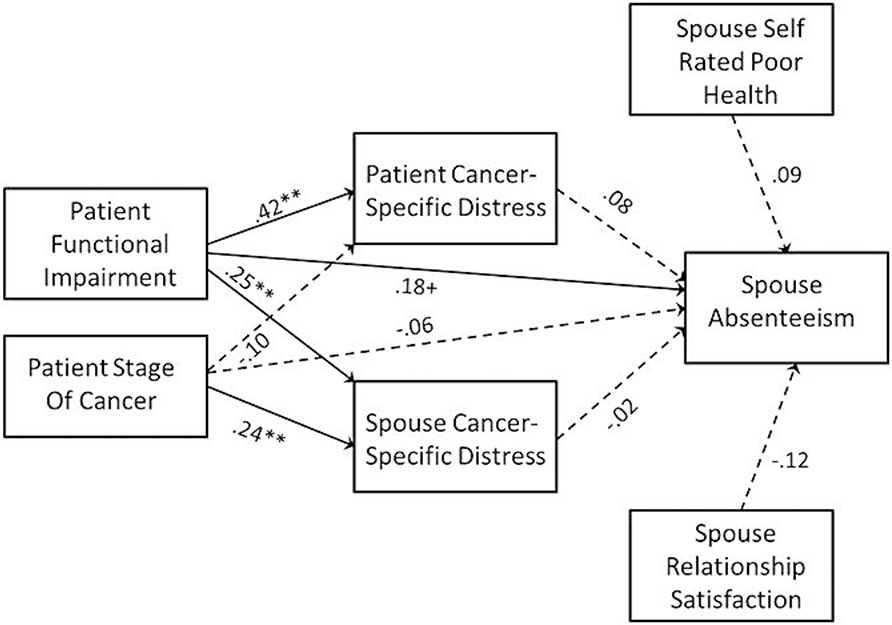
The mediational model predicting spouse work absenteeism is presented in Figure 3. In this case, there was evidence of a modest direct effect of the patient’s functional impairment on the spouse’s absenteeism, b = .042, β = .18, p = .051 (greater impairment was associated with more spouse absenteeism). However, there was no evidence that the effects of either disease severity variable (cancer stage or functional impairment) on work absenteeism were mediated by either partners’ cancer-specific distress.
Figure 3.
Mediational Model Predicting Spouse Absenteeism. Path coefficients are standardized and dashed paths indicate path coefficients that were not statistically different from zero. Higher scores indicate more work absenteeism. + p = .06, * p < .05, ** p < .01
Model predicting spouse work performance
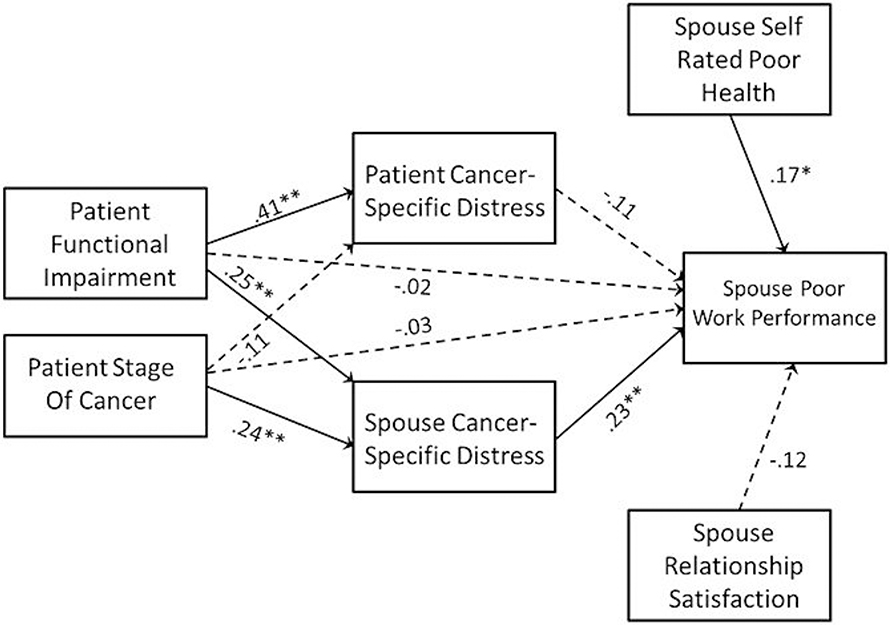
The mediational model predicting spouse work performance is presented in Figure 4. Results indicated an indirect effect of the patient’s cancer stage on spouse’s work performance via the spouse’s cancer-specific distress. The indirect effect of cancer stage on work performance via the spouse’s distress was b = .086, 95% CI = .025–.186, β = .068; 95% CI = .021–.142, p = .007. However, unlike work productivity, there were no direct or indirect effects of the patient’s functional impairment on spouse work performance.
Figure 4.
Mediational Model Predicting Spouse Work Productivity. Path coefficients are standardized and dashed paths indicate path coefficients that were not significantly different from zero. Higher scores on the work productivity scale indicate poorer performance. * p < .05, ** p < .01
Conclusions
Among currently employed husbands of women recently diagnosed with early stage breast cancer, occupational functioning was high. Spouses did not miss a great deal of work, and work productivity and performance were relatively high. The proposed model of a possible mediating effect of patient and spouse distress on the association between the severity of the patients’ illness and spouse occupational functioning was partially supported. With regard to work productivity, greater disease severity was associated with greater spouse cancer-specific distress, which was, in turn, associated with lower spouse productivity. As predicted, spouse cancer-related distress mediated this association. There was a similar finding for poor work performance in that the effects of patient cancer stage were mediated by spouse distress. In contrast, there was no evidence that the effects of the patient’s medical status on spouse work productivity or performance were mediated by the patient’s cancer-specific distress. Spouse marital satisfaction, medical visits, and health conditions did not predict work productivity, but the poorer self-rated health was associated with lower productivity. The proposed model of a mediating effect for patient and spouse distress on the association between cancer illness severity and spouse absenteeism was not supported. Although there was a modest direct effect of the greater patient’s functional impairment on more frequent spouse work absenteeism, there was no evidence that the effect of functional impairment on work absenteeism was mediated by either partners’ cancer-specific distress. In sum, patients with late stage breast cancer and patients who reported higher levels of impairment in daily activities had spouses who reported lower levels of work productivity, and this was in part explained by the fact that the spouses were experiencing greater psychological distress about their partner’s breast cancer.
Our findings extend the limited literature on occupational functioning among spouses of cancer patients. Although work productivity and performance were relatively high and absenteeism was low among those spouses working at the time of their participation in this study, patients experiencing more impairment from the disease and its treatment were more distressed and their spouses were more distressed about the breast cancer. This distress, at least on the part of the spouse, interfered with work productivity. Prior research has suggested that caregiver anxiety and depressive symptoms are associated with income loss among spouses [22], and our study is consistent with this finding. It is interesting that marital satisfaction was not associated with work productivity. However, poorer marital quality was associated with spouse distress, which is consistent with prior work among spouses of late stage cancer patients [40]. As has been discussed in other studies of spouses providing care to patients with cancer, there is a hidden morbidity among spouse caregivers [40]. Our study extends this literature by evaluating the role of relationship variables and patient and partner cancer distress. Work productivity was adversely affected in a subgroup of spouses, and productivity was associated with the severity of the cancer and spousal distress, along with the spouse’s physical health.
There are a number of limitations in this study that need to be considered. First, the study focused on spouses who were currently working. There was a large subgroup of spouses who were retired or not working in the past month: 67% of the spouses completing the survey were not currently working. Focusing on the group of employed spouses was necessary for the current analyses of occupational functioning. However, the sample was biased toward more physically healthy spouses (e.g., spouses who were healthy enough to work), as well as toward spouses for whom caregiving responsibility was not so great that they could continue to work. Second, the study focused solely on husbands. There are significant gender differences in caregiving roles and responsibilities as well as distress responses. Research indicates that wives report more distress [7,41], greater daily activity restriction [42], greater relationship disruption [41,43,44], and greater unmet support needs [41] than male caregivers of cancer patients. Future studies should focus on wives/female partners. Third, this study examined patients who did not have metastatic (stage 3b or 4) breast cancer. Our findings might differ if patients with metastatic stages of disease were included, because physical impairment and support needs would be much greater. Fourth, we did not assess caregiving demands or caregiving burden. It is possible that functional impairment resulted in greater medical treatment demands (e.g., clinic visits, home medical care, assistance with daily activities, and self-care) and that these demands were the reason that impairment was associated with higher levels of spouse distress. Including measures of caregiving demands and burden would be important for future research, as both factors are known correlates of spouse distress [45] and financial burden [46] among caregivers. Our assessment of work productivity was a global measure, and thus, it did not assess the degree to which spouse missed work because of cancer-related care provision or attributed lower productivity at work to cancer-related caregiving or distress about cancer. Fifth, the sample was taken from baseline data from a randomized clinical trial evaluating the efficacy of two couple-focused group interventions. Couples participating in this trial may have been more distressed or exhibit lower levels of relationship satisfaction, which could bias the results of the present analyses. Sixth, the measure of health conditions was not comprehensive, and the severity of conditions was not weighted. Finally, the cross-sectional nature of the study limits the ability to determine causal relationships.
In terms of clinical implications, this study suggests that there is a subgroup of distressed spouses who suffer from a loss of work productivity as a result of the diagnosis and treatment of early stage breast cancer. Clinically, there may be a need to assist these spouses in managing their distress and assist patient and spouse in managing the patient’s functional impairment. Overall, facilitating better psychosocial functioning may ensure that spouse’s work productivity is not adversely affected.
Acknowledgements
We would like to acknowledge Sara Frederick, Devaney Camburn, Lauren Pidgeon, and Tina Gajda for project management, and Kristen Sorice, Emily Richards, Kate Volpicelli, Jenny Iacovone, Jenny Burden, Danielle Ryan, Julianne Lacroce, and Tracy Max for data collection. This work was supported by an R01 grant awarded to Sharon L. Manne (CA-078084).
References
- 1.Fletcher BS, Miaskowski C, Given B, Schumacher K. The cancer family caregiving experience: an updated and expanded conceptual model. Eur J Oncol Nurs 2012;16(4):387–398. DOI: 10.1016/j.ejon.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Ryn M, Sanders S, Kahn K, et al. Objective burden, resources, and other stressors among informal cancer caregivers: a hidden quality issue? Psycho-Oncology 2011;20(1):44–52. DOI: 10.1002/pon.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yabroff KR, Kim Y. Time cost associated with informal caregiving for cancer survivors. Cancer 2009;115(18 Suppl):4362–4373. DOI: 10.1002/cncr.24588. [DOI] [PubMed] [Google Scholar]
- 4.Weitzner MA, Haley WE, Chen H. The family caregiver of the older patient. Hematol Oncol Clin North Am 2000;14(1):269–281. [DOI] [PubMed] [Google Scholar]
- 5.Pinquart M, Sörensen S. Associations of stressors and uplifts of caregiving with caregiver burden and depressive mood: a meta-analysis. J Gerontol B Psychol Sci Soc Sci 2003;58(2):112–128. DOI: 10.1093geronb/58.2.P112. [DOI] [PubMed] [Google Scholar]
- 6.Hinnen C, Ranchor AV, Sanderman R, Snijders TA, Hagedoorn M, Coyne JC. Course of distress in breast cancer patients, their partners, and matched control couples. Ann Behav Med 2008;36(2):141–148. DOI: 10.1007/s12160-008-9061-8. [DOI] [PubMed] [Google Scholar]
- 7.Hagedoorn M, Buunk BP, Kuijer RG, Wobbes T, Sanderman R. Couples dealing with cancer: role and gender differences regarding psychological distress and quality of life. Psycho-Oncology 2000;9(3):232–242. [DOI] [PubMed] [Google Scholar]
- 8.Janda M, Steginga S, Langbecker D, Dunn J, Walker D, Eakin E. Quality of life among patients with brain tumors and their carers. J Psychosom Res 2007;63(6):617–623. [DOI] [PubMed] [Google Scholar]
- 9.Tiedtke C, de Rijk A, Dierckx de Casterlé B, Christiaens MR, Donceel P. Experiences and concerns about ‘returning to work’ for women breast cancer survivors: a literature review. Psycho-Oncology 2010;19(7):667–683. DOI: 10.1002/pon.1633. [DOI] [PubMed] [Google Scholar]
- 10.Ahn C, Cho J, Shin D, et al. Impact of breast cancer diagnosis and treatment on work-related life and factors affecting them. Breast Cancer Res Treat 2009;116:609–616. [DOI] [PubMed] [Google Scholar]
- 11.Balak F, Roelen C, Koopmans P, ten Bere E, Groothoff J. Return to work after early stage breast cancer: a cohort study. J Occup Rehabil 2008;18:267–272. [DOI] [PubMed] [Google Scholar]
- 12.Chirikos TN, Russell-Jacobs A, Cantor AB. Indirect economic effects of long-term breast cancer survival. Cancer Pract 2002;10(5):248–255. [DOI] [PubMed] [Google Scholar]
- 13.Gordon L, Scuffham P, Hayes S, Newman B. Exploring the economic impact of breast cancers during the 18 months following diagnosis. Psycho-Oncology 2007;16(12):1130–1139. [DOI] [PubMed] [Google Scholar]
- 14.Bouknight R, Bradley C, Luo Z. Correlates of return to work for breast cancer survivors. J Clin Oncol 2006;24:345–353. [DOI] [PubMed] [Google Scholar]
- 15.Lauzier S, Maunsell E, De Koninck M, Drolet M, Hébert-Croteau N, Robert J. Conceptualization and sources of costs from breast cancer: findings from patient and caregiver focus groups. Psycho-Oncology 2005;14(5):351–360. [DOI] [PubMed] [Google Scholar]
- 16.Maunsell E, Drolet M, Brisson J, Brisson C, Mâsse B, Deschênes L. Work situation after breast cancer: results from a population-based study. J Natl Cancer Inst 2004;96(24):1813–1822. [DOI] [PubMed] [Google Scholar]
- 17.Stewart DE, Cheung AM, Duff S, et al. Long term breast cancer survivors: confidentiality, disclosure, effects on work and insurance. Psycho-Oncology 2001;10(3):259–263. [DOI] [PubMed] [Google Scholar]
- 18.Northouse LL, Cracchiolo-Caraway A, Appel CP. Psychologic consequences of breast cancer on partner and family. Semin Oncol Nurs 1991;7(3):216–223. [DOI] [PubMed] [Google Scholar]
- 19.Zahlis EH, Shands C. Coming to grips with breast cancer: the spouse’s experience with his wife’s first six months. J Psychosoc Oncol 2010;28(1):79–97. DOI: 10.1080/07347330903438974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollenbeak CS, Short PF, Moran J. The implications of cancer survivorship for spousal employment. J Cancer Surviv 2011;5(3):226–234. DOI: 10.1007/s11764-011-0175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Houtven CH, Ramsey SD, Hornbrook MC, Atienza AA, van Ryn M. Economic burden for informal caregivers of lung and colorectal cancer patients. Oncologist 2010;15(8):883–893. DOI: 10.1634/theoncologist.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosher CE, Champion VL, Azzoli CG, et al. Economic and social changes among distressed family caregivers of lung cancer patients. Support Care Cancer 2013;21(3):819–826. DOI: 10.1007/s00520-012-1585-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherwood PR, Donovan HS, Rosenzweig M, Hamilton R, Bender CM. A house of cards: the impact of treatment costs on women with breast and ovarian cancer. Cancer Nurs 2008;31(6):470–477. DOI: 10.1097/01.NCC.0000339255.75947.a8. [DOI] [PubMed] [Google Scholar]
- 24.Céilleachair AÓ, Costello L, Finn C, et al. Inter-relationships between economic and emotional consequences of colorectal cancer for patients and their families: a qualitative study. BMC Gastroenterol 2012;12:62. DOI: 10.1186/1471-230X-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley S, Sherwood P, Kuo J, et al. Perceptions of economic hardship and emotional health in a pilot sample of family caregivers. J Neurooncol 2009;93:333–42. DOI: 10.1007/s11060-008-9778-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kavanaugh M, Kramer BJ, Walsh MC, Trentham-Dietz A. Factors contributing to economic burden in lung cancer spousal caregivers. Palliat Support Care 2014;1–10. DOI: 10.1017/S1478951514000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segrin C, Badger T, Sieger A, Meek P, Lopez AM. Interpersonal well-being and mental health among male partners of women with breast cancer. Issues Ment Health Nurs 2006;27(4):371–389. [DOI] [PubMed] [Google Scholar]
- 28.Lewis FM, Fletcher KA, Cochrane BB, Fann JR. Predictors of depressed mood in spouses of women with breast cancer. J Clin Oncol 2008;26(8):1289–1295. DOI: 10.1200/JCO.2007.12.7159. [DOI] [PubMed] [Google Scholar]
- 29.Scuffham P, Vecchio N, Whiteford H. Exploring the validity of HPW-based presenteeism measures to estimate productivity losses in the health and education sectors. Med Decis Making 2014;34(1): 127–137. [DOI] [PubMed] [Google Scholar]
- 30.Kessler RC, Barber C, Beck A, et al. The World Health Organization Health and Work Performance Questionnaire. J Occup Environ Med 2003;45(2):156–174. [DOI] [PubMed] [Google Scholar]
- 31.Terry P, Xi M. An examination of presenteeism measures: the association of three scoring methods with health, work life, and consumer activation. Popul Health Manag 2010;13:297. [DOI] [PubMed] [Google Scholar]
- 32.Prasad M, Wahlqvist P, Shikiar R, Shih YC. A review of self-report instruments measuring health-related work productivity. Pharmacoeconomics 2004;22(4):225–244. [DOI] [PubMed] [Google Scholar]
- 33.Kessler RC, Ames M, Hymel PA, et al. Using the World Health Organization Health and Work Performance Questionnaire (HPQ) to evaluate indirect workplace costs of illness. J Occup Environ Med 2004;46:S23–S37. [DOI] [PubMed] [Google Scholar]
- 34.Spanier GB. Measuring dyadic adjustment: new scales for assessing the quality of marriage and similar dyads. J Marriage Fam 1976;38(1):15–28. DOI: 10.2307/350547. [DOI] [Google Scholar]
- 35.Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 36.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med 1979;41(3):209–218. [DOI] [PubMed] [Google Scholar]
- 37.Baider L, Andritsch E, Uziely B, et al. Effects of age on coping and psychological distress in women diagnosed with breast cancer: review of literature and analysis of two different geographical settings. Crit Rev Oncol Hematol 2003;46(1):5–16. [DOI] [PubMed] [Google Scholar]
- 38.Kraemer LM, Stanton AL, Meyerowitz BE, Rowland JH, Ganz PA. A longitudinal examination of couples’ coping strategies as predictors of adjustment to breast cancer. J Fam Psychol 2011;25(6):963–972. DOI: 10.1037/a0025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schag C, Ganz P, Heinrich R. CAncer rehabilitation evaluation system- Short form (CARES SF): a cancer-specific rehabilitation and quality of life instrument. Cancer 1991;68(6): 1406–1413. [DOI] [PubMed] [Google Scholar]
- 40.Braun M, Mikulincer M, Rydall A, Walsh A, Rodin G. Hidden morbidity in cancer: spouse caregivers. J Clin Oncol 2007;25:4829–4834. [DOI] [PubMed] [Google Scholar]
- 41.Ussher JM, Sandoval M, Perz J, Wong WKT, Butow P. The gendered construction and experience of difficulties and rewards in cancer care. Qual Health Res 2013;23(7):900–915. DOI: 10.1177/1049732313484197. [DOI] [PubMed] [Google Scholar]
- 42.Matthews BA. Role and gender differences in cancer-related distress: a comparison of survivor and caregiver self-reports. Oncol Nurs Forum 2003;30(3):493–499. [DOI] [PubMed] [Google Scholar]
- 43.Hagedoorn M, Sanderman R, Bolks HN, Tuinstra J, Coyne JC. Distress in couples coping with cancer: a meta-analysis and critical review of role and gender effects. Psychol Bull 2008;134(1):1–30. DOI: 10.1037/0033-2909.134.1.1hagedoorn. [DOI] [PubMed] [Google Scholar]
- 44.Kayser K, Sormanti M, Strainchamps E. Women coping with cancer. Psychol Women Q 1999;23(4):725–739. DOI: 10.1111/j.1471-6402.1999.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 45.Razani J, Corona R, Quilici J, et al. The effects of declining functional abilities in dementia patients and increases in psychological distress on caregiver burden over a one-year period. Clin Gerontol 2014;37(3):235–252. DOI: 10.1080/07317115.2014.885920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazanec SR, Daly BJ, Douglas SL, Lipson AR. Work productivity and health of informal caregivers of persons with advanced cancer. Res Nurs Health 2011;34(6):483–495. DOI: 10.1002/nur.20461 [DOI] [PMC free article] [PubMed] [Google Scholar]