Abstract
Purpose
To describe spectral-domain OCT (SD-OCT) features, age, gender, and systemic variables that may be used in machine/deep learning studies to identify high-risk patient subpopulations with high risk of progression to geographic atrophy (GA) and visual acuity (VA) loss in the short term.
Design
Prospective, longitudinal study.
Participants
We analyzed imaging data from patients with intermediate age-related macular degeneration (iAMD) (N = 316) enrolled in the Age-Related Eye Disease Study 2 (AREDS2) Ancillary SD-OCT with adequate SD-OCT imaging for repeated measures.
Methods
Qualitative and quantitative multimodal variables from the database were derived at each yearly visit over 5 years. Based on statistical analyses developed in the field of cardiology, an algorithm was developed and used to select person-years without GA on color fundus photography or SD-OCT at baseline. The analysis used machine learning approaches to generate classification trees. Eyes were stratified as low, average, above average, and high risk in 1 or 2 years, based on OCT and demographic features by the risk of GA development or decreased VA by 5+ and 10+ letters.
Main Outcome Measures
New onset of SD-OCT–determined GA and VA loss.
Results
We identified multiple retinal and subretinal SD-OCT and demographic features from the baseline visit, each of which independently conveyed low to high risk of new-onset GA or VA loss on each of the follow-up visits at 1 or 2 years.
Conclusions
We propose a risk-stratified classification of iAMD based on the combination of OCT-derived retinal features, age, gender, and systemic variables for progression to OCT-determined GA or VA loss. After external validation, the composite early end points may be used as exclusion or inclusion criteria for future clinical studies of iAMD focused on prevention of GA progression or VA loss.
Keywords: Classification trees, Geographic atrophy, Machine learning, Prediction, Random forest
Abbreviations and Acronyms: AMD, age-related macular degeneration; AREDS2, Age-Related Eye Disease Study 2; A2A, Ancillary SD-OCT; AUC, area under the curve; CI, confidence interval; ETDRS, Early Treatment Diabetic Retinopathy Study; GA, geographic atrophy; HRF, hyper-reflective foci; iAMD, intermediate age-related macular degeneration; NS, neurosensory; NV, neovascularization; OR, odds ratio; RPE, retinal pigment epithelium; RPEDC, retinal pigment epithelium drusen complex; SDD, subretinal drusenoid deposit; SD-OCT, spectral-domain OCT; VA, visual acuity
Translation of pathophysiological insight of intermediate age-related macular degeneration (iAMD) to clinical studies requires better understanding of the timeline of visible biomarkers that manifest throughout the stages of iAMD. Despite the widespread use of spectral-domain OCT (SD-OCT), which is invaluable in monitoring late age-related macular degeneration (AMD), there is an insufficient understanding of the combination of specific structural biomarkers that predict progression to late disease, either choroidal neovascularization (NV) or geographic atrophy (GA).
The interplay among patterns of injury, SD-OCT findings, and systemic health is also not well understood. Thus, there is a critical need for cost-effective, incisive classification systems for iAMD; these will require integration of SD-OCT findings that have historically been studied in isolation. Spectral-domain OCT imaging of retinal layers and drusen substructures characterizes pathologies and progressive changes in AMD.1 The investigation of the combination of imaging biomarkers associated with pathways of progression to GA will enable cost-effective, clinical studies based on disease pathobiology.
An extremely valuable dataset of iAMD eyes that can be used for identification of SD-OCT biomarkers of disease progression is the Age-Related Eye Disease Study 2 (AREDS2) Ancillary SD-OCT (A2A) study. It is an observational, ancillary, prospective study of a subset of eyes from AREDS2 that included a group of control eyes of age-matched adults. The AREDS2 trial was a multicenter, prospective, randomized trial conducted to test the effect of oral nutritional supplements on the progression of AMD on color photographs. The A2A study provided the largest and most comprehensive longitudinal dataset of SD-OCT images, demographic, and systemic information in iAMD. The study included 316 patients with 1499 visits in 7.4 years, with a mean follow-up of 4.3 years. Using this dataset, our group has previously shown that abnormalities in the retinal pigment epithelium-drusen complex (RPEDC) volume, retinal pigment epithelium (RPE) abnormal thinning volume, drusen substructures, and hyper-reflective foci (HRF) axial distance severity score were preatrophic markers that predicted the 2-year progression to GA as defined by color photographs.2, 3, 4 We also developed a risk-assessment model based on age and SD-OCT features for progression to color photography–determined GA over up to 5 years.4 Because of recent advances in multimodal imaging of GA, the retina community is progressing toward a modern definition of GA based on high-resolution SD-OCT,5, 6, 7, 8, 9 which allows early GA identification before lesions are apparent on color fundus images. Thus, this analysis adopted an updated definition of GA based on SD-OCT.1,3,4
With the use of the dataset from the A2A trial, the goal of this work was to determine short-term (1- and 2-year) SD-OCT predictors of GA and visual loss in iAMD patients. We hypothesized that from a comprehensive dataset with SD-OCT–based imaging at multiple intervals in eyes with iAMD, we can identify the most important predictors of visual acuity (VA) loss or GA and create cluster predictors to classify iAMD eyes into categories of likelihood to progress to these end points. Next, we sought to estimate the rate of progression from iAMD to the end point of GA or visual loss using a combination of SD-OCT imaging biomarkers, demographic, and clinical factors.
Methods
Database
This analysis used longitudinal data from A2A SD-OCT (ClinicalTrials.gov identifier NCT00734487), a prospective, observational, ancillary study of a subset of eyes from AREDS2.10 The AREDS2 study (ClinicalTrials.gov identifier NCT00345176) was a multicenter, randomized trial that studied the effects of oral nutritional supplements on the progression of AMD on color fundus photography.2 The A2A SD-OCT study followed 316 participants with AMD from 4 AREDS2 clinical sites in the United States (National Eye Institute, Duke Eye Center, Devers Eye Institute, and Emory Eye Center) as described previously.3,4 The study was approved by the Institutional Review Board at each of the 4 clinical sites. Informed written consent was obtained before enrollment from each study participant, and the research protocol followed tenets of human research as presented in the Declaration of Helsinki. Data were collected, stored, and managed in compliance with Health Insurance Portability and Accountability Act guidelines.
All A2A participants had been determined to have iAMD with (1) bilateral large drusen ≥125 μm or noncentral GA (no advanced AMD) or (2) large drusen or noncentral GA in 1 eye and “advanced” AMD (NV or central GA) in the fellow eye.2 The eyes could have an AREDS Simple Scale Score of 2, 3, or 4 and were required to lack “advanced” AMD as defined in AREDS and AREDS2 as the presence of NV or central GA.2,11 Only study eyes with large drusen without GA were included in the current study.
The A2A dataset consisted of 316 participants (aged 51–88 years) with at least 1 iAMD eye at baseline, 301 at year 1, 285 at year 2, 267 at year 3, 167 at year 4, and 77 at year 5 or beyond, with demographics (gender, age, and race) and annual examination, systemic, and eye health data for the period of the AREDS2 study (smoking, use of statins, acetaminophen, aspirin, nonsteroidal anti-inflammatory drugs, cataract surgery, weight, diet report, diabetes status, AMD treatment, and supplement use). To improve the long-term analysis of AMD features beyond the 5 years in the AREDS2 trial, subjects enrolled in the A2A SD-OCT study were asked to return for an additional extension visit that allowed for data capture for up to 7.4 years. Annual SD-OCT imaging was captured with the same investigational tabletop SD-OCT imaging system (Bioptigen) at all 4 sites using the imaging specifications described previously.3 Spectral-domain OCT volume scans were de-identified and graded using the Duke Optical Coherence Tomography Retinal Analysis Program.3,12 Certified, masked graders in the Duke Advanced Research in SS/SD-OCT Imaging laboratory analyzed the SD-OCT scan volumes of study and fellow eyes for acceptable quality and for the presence of qualitative and quantitative SD-OCT measures of macular pathology. These SD-OCT variables (hyperreflective foci axial distance score, subretinal drusenoid deposits [SDDs], RPE atrophy, photoreceptor loss, neurosensory (NS) retina volume, RPE drusen complex volume, drusen volume, RPE drusen complex abnormal thinning volume, and choroidal thickness) have been previously described in detail.3,4 In concordance with recent definitions by other groups,5, 6, 7, 8, 9 OCT-GA was defined as the presence of all 3 of the following criteria: (1) "RPE atrophy or absence," (2) "choroid hyper-transmission," and (3) "outer plexiform layer dipping toward the RPE," together over an area that is at least 175 μm in at least 1 direction. Each of the 3 criteria was defined previously.1,3,4 To evaluate the reproducibility of SD-OCT gradings, 2 independent graders graded a randomly selected subset of 63 study eyes at various times. Based on the Fleiss’ kappa statistic, good to excellent agreement was found between the independent graders3,4 for the SD-OCT findings used in our work.
The variables included as inputs for the model presented in this article consisted of age, gender, systemic variables such as aspirin use and smoking status, and SD-OCT grading variables.1,3,4 A complete list of variables is included in Supplementary Table S1 (available at www.ophthalmologyscience.org); these variables were defined in prior publications.1,3,4
Machine Learning Analytical Approach
Our methods incorporated subsequent repeated measures of the risk factors to enable a more confident classification of risk of progression. The analytical scaffold structures data were based on the person-years methods that have been used and validated since the Framingham Heart Study.13 The approach in this method treats each observation interval as a small follow-up study. Measurements of variables were made at the interval’s baseline and used to predict an outcome event in the follow-up interval. Observations over several intervals are pooled into a single sample (pool). Compared with the previous analyses,1,3,4 in which the prediction of disease is based only on the baseline measurements, this method incorporates subsequent repeated measures of the risk factors and enables more confident estimation of the turning points of progression.
We used the merged iAMD database from the prospective A2A SD-OCT study, which was cross-referenced, combined, and audited (K.S., C.T.). This study used longitudinal data from the 316 study participants with analyses across 1499 visits over 7.4 years. Further data handling, statistical computing, modeling, and algorithms were executed using custom codes (S.H., D.B.) in the R language and environment (free software, version 3.4.2).
By using SD-OCT grading variables with which to build and implement the analytical methodology, a separate database was built for each outcome. The outcomes of interest were GA, vision loss of 5+ letters, vision loss of 10+ letters, and combination of GA and vision loss of 5+ and 10+ letters. Each database was appropriately structured per the patient-year approach before feeding to the machine learning algorithm. From all visits of every eye (from baseline to maximum follow-up year 7 when present), every combination of 2 visits (referred to here as “visit pairs”) was used to define an interval: The earlier visit is the starting point or relative baseline, and the later visit is the outcome assessment time point. The interval of observation within each visit pair was defined by the length of follow-up: Interval duration of interest was approximately 1 year or 2 years. For each of these follow-up intervals, we extracted a separate database for the 1-year pool and the 2-year pool. For each outcome, visit pairs were selected for inclusion in the analysis pool only if the relative baseline visit in that pair/interval was free of the outcome of interest.
Next, each database for a combination of pooled interval and outcome of interest (e.g., new GA over 1-year interval or vision loss of 10+ letters over 2-year interval) was fed into the machine learning algorithm to explore time relationships between the group of clinical health variables and SD-OCT features of iAMD, that had been individually explored, and the outcome of progression. As such, machine learning algorithms evaluated the outcome for composite precursors at each follow-up interval of interest. For each outcome, 2 decision trees were run, 1 for each interval pool (1-year and 2-year). Decision trees consist of recursive partitioning on binary decisions, ensuring generalization to a holdout sample. The final result was a validated decision tree for each interval pool.
We performed 5-fold cross-validation to tune the hyperparameters of our machine learning model and trained a model with the tuned parameters on the full dataset afterward. In the 5-fold cross-validation, each mutually exclusive subset or "fold" was based on a single testing set, which was composed of 20% of participants who were exclusive to the fold. The resulting receiver operating characteristic was used to choose the operating point for the reported sensitivity and specificity to minimize the Index of Union, which is equal to the number of false predictions (false-negatives and false-positives) in the validation set.14
Machine Learning Output
In classification or decision trees, each branching point represents the decision that separates the data between the classes most evenly. One can follow the branches in the tree to understand what successive decisions lead to the most distal branches, which are termed “nodes.” A node is a combination of variables that defines a certain category of risk, leading to a particular outcome. Eyes were stratified, based on node risk, by the risk of GA development or VA loss as low (< 15% risk), average (15%–25%), above average (26%–45%), and high risk (> 45%) in 1 or 2 years. The percentage cutoffs for OCT-GA were chosen a priori based on the 4-year incidence of new GA of approximately 20%;15 therefore, conservatively we expected an incidence of GA < 15% at 2 years and labeled this as "low risk." According to prior research, the estimates for vision loss of 10 to 15 letters over 2 to 3 years range from approximately 10%–15% for nonatrophic (early and intermediate) AMD16 and 40%–50% for atrophic AMD.17, 18, 19 As a result, we proposed the categorization of VA loss of 5 or 10 letters over 1 or 2 years as < 15% low, 15%–25% average, 26%–45% above average, and > 45% high.
As part of the final processing, the trees were systematically truncated at the level above any branching point that yielded small sample size in the node (K.S., E.M.L.). The criteria used to truncate were (1) if the lower-risk arm below the branching point (where the distribution of rare events is of interest) had fewer than 5 events or (2) if the higher-risk arm below the branching point (where the distribution of frequent events is of interest) had fewer than 5 nonevents. The rationale for choosing 5 events when truncating trees was due to the fact that for a chi-square test to be valid, the expected number of observations is required to be > 5 per cell. Nodes or end-branches of each tree were characterized by the subgroup of characteristics that defines the corresponding group of eyes. Accordingly, nodes were risk-stratified based on the 4 risk categories described. Furthermore, the rates of events in each node were extracted into contingency 2 × 2 tables in MS Office Excel and compared with the rest of the nodes combined. Based on contingency tables, odds ratios (OR) were calculated manually using MS Office Excel, and chi-square P values were calculated using the online calculator tool http://vassarstats.net/ (K.S.).
Summary of Methods
This study uses the existing database from the A2A study10 in which SD-OCT images were captured from AMD subjects annually for 7 years. The data points used in this study were derived from 1 eye from each participant and compiled into the master dataset. The variables were derived from grading of SD-OCT images for certain features, and for the outcome of interest, GA as defined on SD-OCT. The master dataset was processed using a Machine Learning algorithm, using assumptions and methods extrapolated from the Framingham Heart Study.13 The result of this processing was 4 longitudinal cohort datasets, each intended for a separate follow-up analysis: the 1-year cohort, the 2-year cohort, the 3-year cohort, and the 4-year cohort.
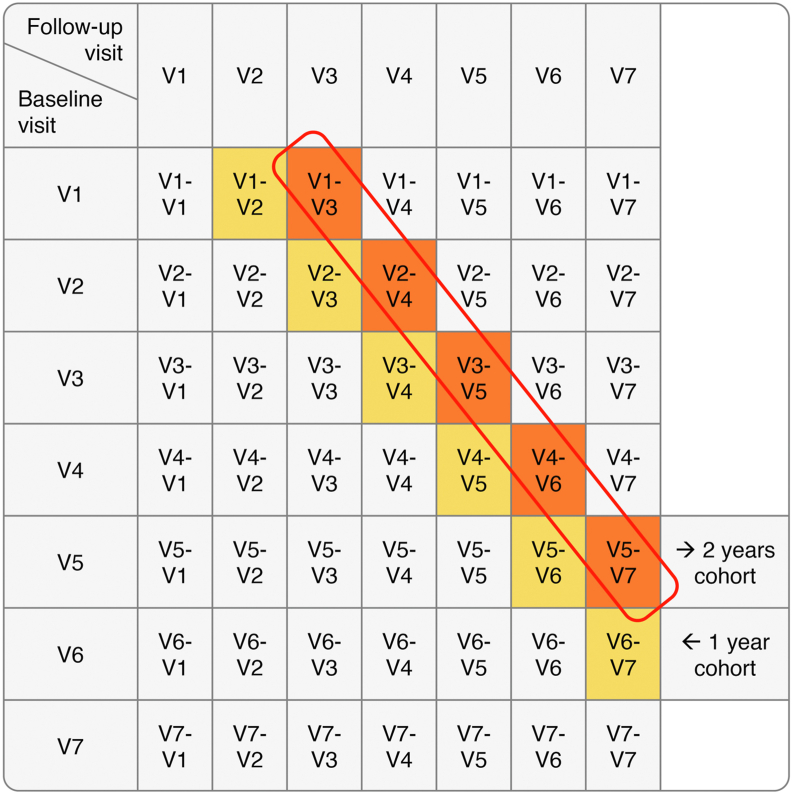
In the master dataset, each participant should have 7 annual data points (visits V1 to V7), assuming a nil attrition rate. Figure 1 shows an illustrative example. If it satisfies the inclusion/exclusion criteria, each of these data points (V1 to V7) may be considered as a baseline visit (Fig 1, rows). Each baseline visit was then paired with each and any of the visits that occur later (Fig 1, columns). The resulting pairs form the follow-up intervals (Fig 1, the 21 cells highlighted). Each follow-up interval was treated as if it were an independent participant (also called person-year) in the respective longitudinal cohort. For example, only the “2-year follow-up intervals” contribute to the “2-year cohort.” Each longitudinal cohort dataset was fed into a recursive partitioning analysis. The output of such analysis was in the form of a decision tree. The end nodes of the decision tree are subgroups of eyes with similar features that convey a certain risk for progression to the outcome (GA or VA loss).
Figure 1.
Example illustrating key methodology. In the master dataset, each participant should have 7 annual data points (visits V1 to V7). Each can be considered as a baseline visit (rows). Each baseline visit was then paired with each and any of the subsequent visits (columns). The resulting pairs form the follow-up intervals (21 cells highlighted).
Results
Earlier reports on analyses from the A2A study presented results related to demographics such as age and gender, systemic variables such as aspirin use and smoking status, and results related to quantitative modeling on OCT.1,3,4
Classification Trees for SD-OCT−Determined GA
One Year
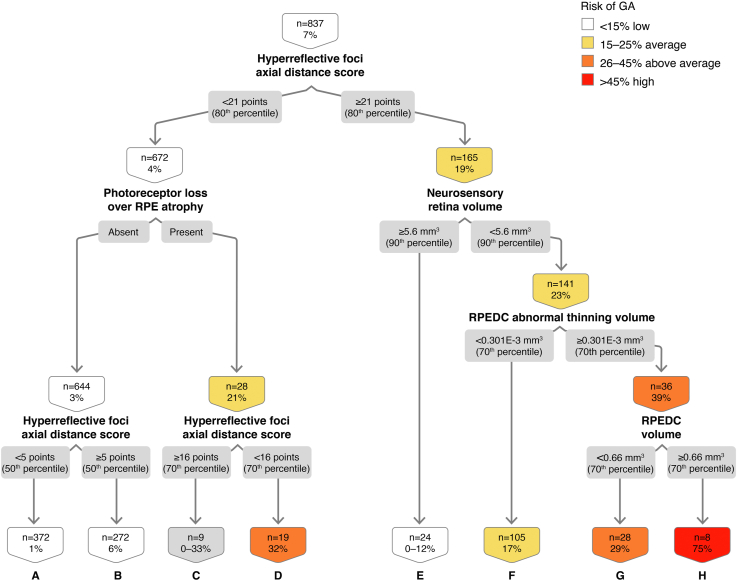
In the classification tree for OCT-determined GA at 1 year, using 837 eye 1-year intervals, we found that HRF axial distance score, a measure of HRF severity driven by location toward the inner retina and number of HRF,16 drives the classification tree (Fig 2). The presence of HRF is associated with a 6.93% likelihood of conversion to GA in 1 year. The combination of high HRF axial distance score (≥ 80th percentile of the baseline values), NS retinal volume < 90th percentile, high RPEDC abnormal thinning volume (≥ 70th percentile), and high RPEDC volume (≥ 70th percentile), confers a 75% risk of OCT-determined GA at 1 year (node H). The OR of GA development over 1 year in the presence of all these features is 44.83 (Table 1; 95% confidence interval [CI], 8.83–227.60, P < 0.01).
Figure 2.
Classification tree for spectral-domain (SD)-OCT geographic atrophy (GA) at the 1-year interval. RPE = retinal pigment epithelium; RPEDC = retinal pigment epithelium drusen complex.
Table 1.
SD-OCT GA at 1- and 2-Year Intervals
|
Node |
1-Year Interval |
2-Year Interval |
||||||
|---|---|---|---|---|---|---|---|---|
|
N Count |
GA Count (%) |
OR (95% CI) |
P Value |
N Count |
GA Count (%) |
OR(95% CI) |
P Value |
|
| A | 372 | 4 (1) | 0.08 (0.03–0.23) | <0.01 | 330 | 7 (2) | 0.14 (0.06–0.32) | <0.01 |
| B | 272 | 16 (6) | 0.78 (0.43–1.41) | 0.41 | 165 | 8 (5) | 0.55 (0.25–1.21) | 0.14 |
| C | 9 | 0 (<33)∗ | - | - | 26 | 0 (<12)∗ | - | - |
| D | 19 | 6 (32) | 6.80 (2.48–18.62) | <0.01 | 19 | 9 (47) | 13.52 (5.19–35.27) | <0.01 |
| E | 24 | 0 (<12.5)∗ | - | - | 31 | 3 (10) | 1.35 (0.39–4.61) | 0.64 |
| F | 105 | 18 (17) | 3.58 (1.97–6.52) | <0.01 | 28 | 10 (36) | 8.45 (3.64–19.61) | <0.01 |
| G | 28 | 8 (29) | 6.07 (2.55–14.47) | <0.01 | 11 | 0 (<27)∗ | - | - |
| H | 8 | 6 (75) | 44.83 (8.83–227.60) | <0.01 | 18 | 10 (56) | 19.36 (7.21–51.96) | <0.01 |
| Total | 837 | 58 (7) | 628 | 47 (7) | ||||
CI = confidence interval; GA = geographic atrophy; OR = odds ratio; SD-OCT = spectral-domain OCT.
Proportions in cells with a count of zero (out of N observations) are estimated using the Rule of 3 whereby the 95% CI for the proportion (%) is between 0 and 3/N.
This classification tree reveals that over the 5 years analyzed, we captured 58 events, defined as study eyes with iAMD that converted to GA. Because 58 of the 316 iAMD eyes that were enrolled in the A2A study converted to GA, the incidence of GA conversion is 18.35% in 5 years, or 3.67% per year, consistent with previous literature that used color photos for GA determination.20 The out-of-sample area under the curve (AUC) on the cross-validated model is 0.80 (80% chance that the model will have a better aggregated classification performance than classification at random), with sensitivity of 0.72 and specificity of 0.78.
Two Years
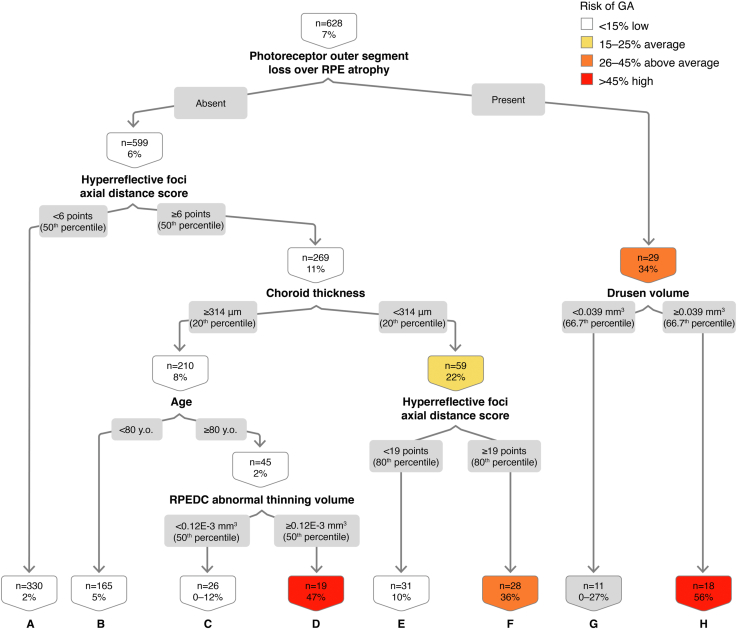
In the classification tree for OCT-determined GA at 2 years, analyzing 628 distinct 2-year intervals during the study duration for all the study eyes, we demonstrate that the driving variable at the top of the decision tree is photoreceptor outer segment loss over RPE atrophy (Fig 3). The combination of absence of photoreceptor outer segment loss over RPE atrophy, HRF axial distance score ≥ 50th percentile, choroid thickness ≥ 20th percentile, age ≥ 80 years, and RPEDC abnormal thinning volume ≥ 50 percentile confers a 47% risk of OCT-determined GA at 2 years (Fig 3; node D). The OR of GA development in the presence of all these features compared with the absence of these features is 13.52 (Table 1; 95% CI, 5.19–35.27, P < 0.01). The out-of-sample AUC on the cross-validated model is 0.75, with sensitivity of 0.65 and specificity of 0.71.
Figure 3.
Classification tree for spectral-domain (SD)-OCT geographic atrophy (GA) at the 2-year interval. RPE = retinal pigment epithelium; RPEDC = retinal pigment epithelium drusen complex.
Classification Trees for VA Loss
VA Loss ≥ 5 Letters at 1 Year
In the classification tree for VA loss ≥ 5 Early Treatment Diabetic Retinopathy Study (ETDRS) letters at 1 year, the combination of thinner choroid (< 66.6th percentile of baseline values) and poorer contralateral eye VA (< 50th percentile) confers a 27% risk of having VA loss ≥ 5 letters at 1 year (Fig 4; node C). Conversely, a thicker choroid appears to be protective, conferring an only 13% risk of VA loss ≥ 5 letters at 1 year (Fig 4; node A). This classification tree has a small number of branches, because trees were truncated at the level above any branching point that yielded fewer than 5 events in the low-risk arm or high-risk arm. The out-of-sample AUC on the cross-validated model is 0.76, with sensitivity of 0.73 and specificity of 0.68.
Figure 4.
Classification tree for visual acuity (VA) loss ≥ 5 letters at the 1-year interval.
VA Loss ≥ 5 Letters at 2 Years
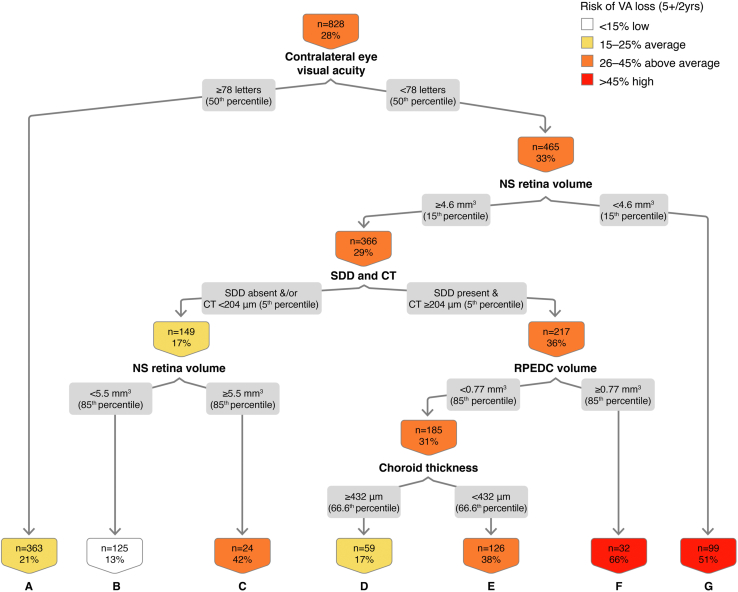
In the classification tree for VA loss ≥ 5 letters during a 2-year interval (Fig 5), using 828 eye 2-year intervals, we found that contralateral eye VA strongly drives the classification tree. Contralateral eye VA ETDRS score < 78 letters (Snellen equivalent 20/30) is associated with a 28% likelihood of VA loss ≥ 5 letters at 2 years. The combination of contralateral eye VA < 78 letters, NS retina volume ≥ 15th percentile, SDDs, and RPEDC volume ≥ 85th percentile confers a 66% risk of VA loss ≥ 5 letters at 2 years (node F). The OR of VA loss ≥ 5 letters in the presence of all these features is 5.29 (Table 2; 95% CI, 2.51–11.16, P < 0.01). The out-of-sample AUC on the cross-validated model is 0.67, with sensitivity of 0.68 and specificity of 0.61.
Figure 5.
Classification tree for visual acuity (VA) loss ≥ 5 letters at the 2-year interval. CT = choroidal thickness; NS = neurosensory; RPEDC = retinal pigment epithelium drusen complex; SDD = subretinal drusenoid deposit.
Table 2.
VA Loss ≥ 5 Letters at 1- and 2-Year Intervals
| 1-Year Interval | 2-Year Interval | |||||||
|---|---|---|---|---|---|---|---|---|
| Node |
N Count |
GA Count (%) |
OR (95% CI) |
P Value |
N Count |
GA Count (%) |
OR(95% CI) | P Value |
| A | 322 | 42 (13) | 0.50 (0.35–0.72) | <0.01 | 363 | 77 (21) | 0.54 (0.39–0.74) | <0.01 |
| B | 272 | 45 (17) | 0.73 (0.51–1.05) | 0.09 | 125 | 16 (13) | 0.33 (0.19–0.57) | <0.01 |
| C | 512 | 136 (27) | 2.11 (1.56–2.85) | <0.01 | 24 | 10 (42) | 1.87 (0.82–4.28) | 0.14 |
| D | - | - | - | - | 59 | 10 (17) | 0.50 (0.25–1.01) | 0.05 |
| E | - | - | - | - | 126 | 48 (38) | 1.73 (1.16–2.58) | 0.01 |
| F | - | - | - | - | 32 | 21 (66) | 5.29 (2.51–11.16) | <0.01 |
| G | - | - | - | - | 99 | 50 (51) | 3.07 (2.00–4.71) | <0.01 |
| Total | 1106 | 223 (20) | 828 | 232 (28) | ||||
CI = confidence interval; GA = geographic atrophy; OR = odds ratio; VA = visual acuity.
VA Loss ≥ 10 Letters at 1 Year
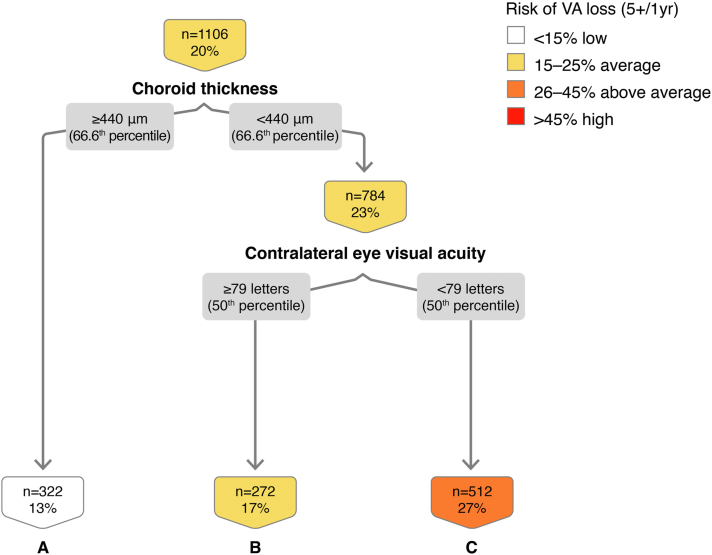
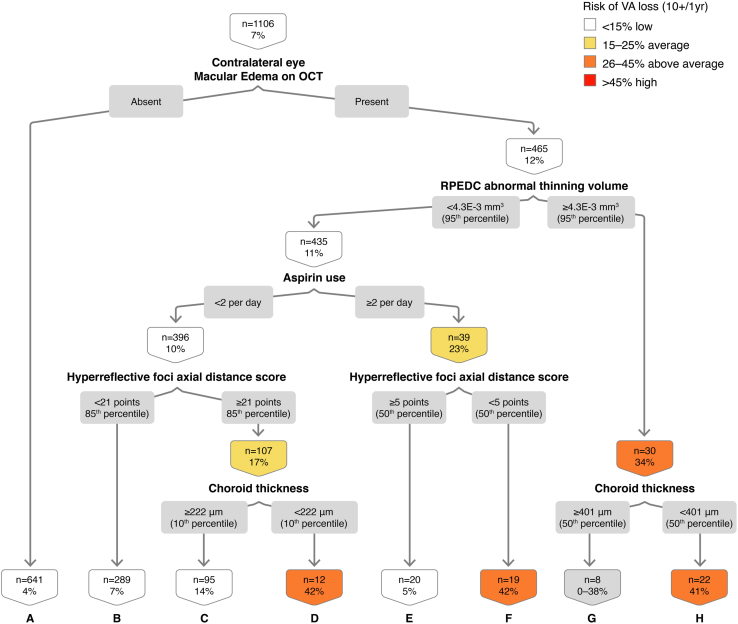
In the decision tree for VA loss ≥ 10 letters at 1 year, using 1106 eye-year events, we found that contralateral (fellow) eye macular edema on OCT is important in this classification (Fig 6). Macular edema on OCT in the contralateral eye is associated with a 7.4% likelihood of VA loss ≥ 10 ETDRS letters at 1 year. The combination of contralateral eye macular edema on OCT, standard RPEDC abnormal thinning volume (< 95th percentile), lower aspirin use (< 2 per day), high HRF axial distance score (≥ 85th percentile), and thin choroid (< 10th percentile) confers a 42% risk of VA loss ≥ 10 letters at 1 year (Fig 6; node D). The out-of-sample AUC on the cross-validated model is 0.65, with sensitivity of 0.60 and specificity of 0.59. Absence of contralateral eye edema on OCT (Fig 6; node A) appears to be protective in this classification analysis (4.1% risk, OR, 0.31, 95% CI, 0.19–0.50, P < 0.01).
Figure 6.
Classification tree for visual acuity (VA) loss ≥ 10 letters at the 1-year interval. RPEDC = retinal pigment epithelium drusen complex.
VA Loss ≥ 10 Letters at 2 Years
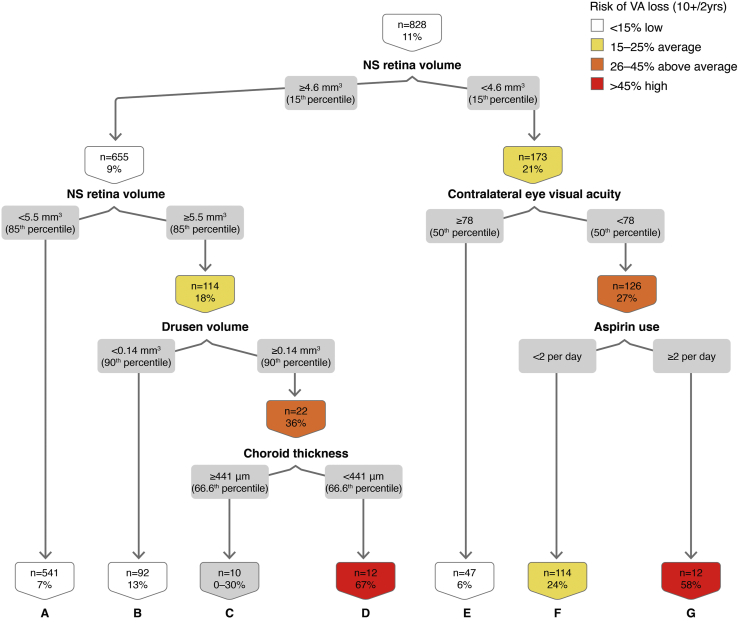
Using 828 eye 2-year intervals, we noted that NS retina volume is at the first branching point in this classification tree (Fig 7). The combination of NS retina volume ≥ 85th percentile, high drusen volume (≥ 90th percentile), and standard choroid thickness (< 66.6th percentile) confers 67% risk of VA loss ≥ 10 letters at 2 years (Fig 7; node D). The OR for VA loss ≥ 10 letters at the 2-year interval in the presence of all these features is 17.20 (Table 3; 95% CI, 5.07–58.32, P < 0.01). The combination of thin NS retina volume < 15th percentile, poorer contralateral eye VA (< 50th percentile), and high aspirin use (≥ 2 per day) confers a 58% risk of VA loss ≥ 10 letters at the 2-year interval (Fig 7; node G), whereas aspirin use of < 2 per day confers 24% risk (node F). The out-of-sample AUC on the cross-validated model is 0.64, with sensitivity of 0.58 and specificity of 0.56.
Figure 7.
Classification tree for visual acuity (VA) loss ≥ 10 letters at the 2-year interval. NS = neurosensory.
Table 3.
VA Loss ≥10 Letters at 1- and 2-Year Intervals
| 1-Year Interval | 2-Year Interval | |||||||
|---|---|---|---|---|---|---|---|---|
| Node |
N Count |
GA Count (%) |
OR (95% CI) |
P Value |
N Count |
GA Count (%) |
OR(95% CI) |
P Value |
| A | 641 | 26 (4) | 0.31 (0.19–0.50) | <0.01 | 541 | 36 (7) | 0.29 (0.18–0.45) | <0.01 |
| B | 289 | 20 (7) | 0.91 (0.54–1.53) | 0.71 | 92 | 12 (13) | 1.21 (0.63–2.32) | 0.56 |
| C | 95 | 13 (14) | 2.16 (1.15–4.08) | 0.02 | 10 | 0 (<30)∗ | - | - |
| D | 12 | 5 (42) | 9.43 (2.93–30.42) | <0.01 | 12 | 8 (67) | 17.20 (5.07–58.32) | <0.01 |
| E | 20 | 1 (5) | 0.65 (0.09–4.94) | 0.68 | 47 | 3 (6) | 0.52 (0.16–1.72) | 0.29 |
| F | 19 | 8 (42) | 9.96 (3.89–25.51) | <0.01 | 114 | 27 (24) | 3.05 (1.85–5.03) | <0.01 |
| G | 8 | 0 (<38)∗ | - | - | 12 | 7 (58) | 11.88 (3.69–38.26) | <0.01 |
| H | 22 | 9 (41) | 9.59 (3.97–23.17) | <0.01 | - | - | - | - |
| Total | 1106 | 82 (7) | 828 | 93 (11) | ||||
CI = confidence interval; GA = geographic atrophy; OR = odds ratio; VA = visual acuity.
Proportions in cells with a count of zero (out of N observations) are estimated using the Rule of 3 whereby the 95% CI for the proportion (%) is between 0 and 3/N.
Classification Trees for Composite End Points (OCT-GA and Visual Loss)
Next, we attempted to develop classification trees for the combination of structural (OCT-GA) and functional (visual loss) end points. The analyses of OCT-GA with VA loss ≥ 5 letters or ≥ 10 letters at 1 year and OCT-GA with VA loss ≥ 10 letters at 2 years were not feasible because of a low number of events for the combined outcome.
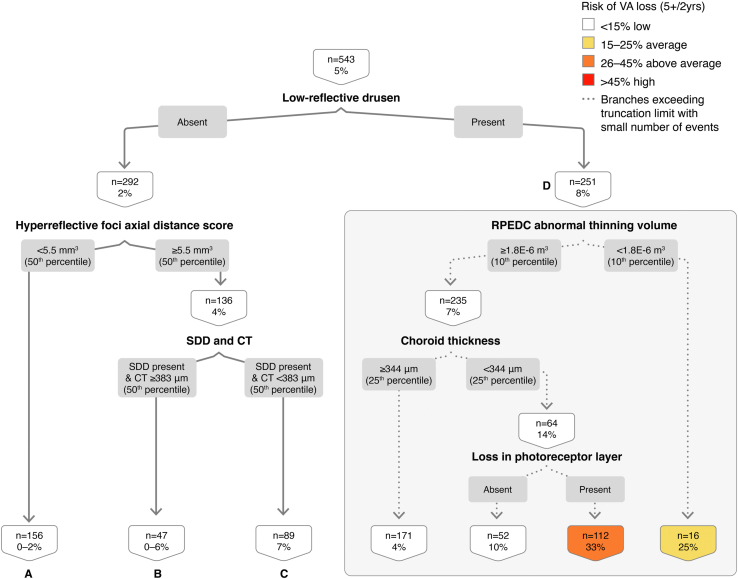
In the classification tree for OCT-determined GA with VA loss ≥ 5 letters at the 2-year interval (Fig 8), using 543 eye 2-year intervals, we found that the presence of low-reflective drusen drives the classification tree and is associated with an 8% risk of GA development with VA loss ≥ 5 letters at 2 years (node D). The OR of GA development with VA loss of ≥ 5 letters in the presence of low-reflective drusen is 4.13 (Table 4; 95% CI, 1.63–10.45, P < 0.01). By contrast, the absence of low reflective drusen and low HRF score < 50th percentile are protective for GA development with VA loss (Fig 8; node A: 0% risk, OR, 0.04, 95% CI, 0–0.72, P = 0.03).
Figure 8.
Classification tree for spectral-domain (SD)-OCT geographic atrophy (GA) with visual acuity (VA) loss ≥ 5 letters at the 2-year interval. CT = choroidal thickness; RPEDC = retinal pigment epithelium drusen complex; SDD = subretinal drusenoid deposit.
Table 4.
SD-OCT GA with VA Loss ≥ 5 Letters at the 2-Year Interval
| 2-Year Interval | ||||
|---|---|---|---|---|
| Node |
N Count |
GA Count (%) |
OR (95% CI) |
P Value |
| A | 156 | 0 (<2)∗ | - | - |
| B | 47 | 0 (<6)∗ | - | - |
| C | 89 | 6 (7) | 1.57 (0.61–4.02) | 0.35 |
| D | 251 | 20 (8) | 4.13 (1.63–10.45) | <0.01 |
| Total | 543 | 26 (5) | ||
CI = confidence interval; GA = geographic atrophy; OR = odds ratio; SD-OCT = spectral-domain OCT; VA = visual acuity.
Proportions in cells with a count of zero (out of N observations) are estimated using the Rule of 3 whereby the 95% CI for the proportion (%) is between 0 and 3/N.
Discussion
The failure of prior interventions for GA may be due to gaps in our current understanding of the pathobiology of dry AMD, patient selection, application of the therapy too late in the disease process, and a paucity of useful alternative clinical trial end points. Future therapeutic trials are likely to be most readily accepted and promising in the early and intermediate stages of dry AMD, in which an intervention would be applied before irreversible loss of vision. The first challenge, that of determining vision function end points useful in early-intermediate AMD patients with the essentially unaffected best-corrected VA, is currently being addressed by natural history studies such as that recently completed at Duke University,20 the International AMD Ryan Initiative Study20 led by the National Eye Institute and the MACUSTAR initiative in Europe.21 The second challenge, that of patient selection, is just as crucial for the success of future trials of iAMD, given the numerous historical failures to date and the need for shorter-term clinical trials. Thus, the goal of the current work was to begin to address these limitations and to formulate recommendations for future clinical trials in terms of patient selection.
Identification of individual, specific SD-OCT patterns that can predict advancing disease was the main aim of several studies analyzing data from the A2A SD-OCT study. In iAMD, drusen-related reflectivity patterns3 and HRF1,22 were found to represent early indicators of disease progression. New-onset GA as defined on color photographs was preceded by SD-OCT findings of atypical drusen,3 OCT-reflective drusen substructures,1 HRF,22,23 and quantitative SD-OCT measurements.24 Prior work by Toth et al revealed that the strongest biomarkers of progression were age, HRF, a greater RPEDC abnormal thinning volume and RPE layer atrophy.4 These findings were recapitulated in the current work on short-term predictors of progression to SD-OCT-determined GA in iAMD.
Machine learning analyses applied on longitudinal OCT imaging data have recently emerged as powerful approaches for predictive modeling for a plethora of ophthalmic applications, including in AMD. Examples include drusen regression in early-intermediate AMD,25 anti-vascular endothelial growth factor treatment response26 and progression to late AMD stages,27 and progression of GA.28 Bogunovic et al25 used a longitudinal dataset from a prospective, observational study of OCT images from 61 eyes with early-intermediate AMD from 38 patients to propose a predictive model of drusen regression. The authors developed a machine learning method based on survival analysis and leave-1-patient-out cross-validation to predict individual druse regression (AUC 0.75 within the first 2 years). This pilot study, although the first to allow a personalized and reproducible prediction of drusen regression, was limited by the small sample size, lack of inclusion of SDDs in the model, and absence of a data-driven link between drusen regression to progression and advanced AMD stages. Using image features characterizing drusen area, volume, height, and reflectivity in a dataset of 330 eyes in 244 patients, de Sisternes et al27 developed a statistical model that predicted the conversion from early-intermediate AMD to exudative AMD in less than 5 years. Another model based on quantitative characteristics of GA lesions was developed in a smaller study of 118 SD-OCT scans from 38 eyes of 29 patients to estimate future growth of GA at a given time (median follow-up of 2.25 years). The SD-OCT biomarkers that were most predictive of future regions of GA growth were thicknesses or SDD, changes in reflectivity, and loss of outer retinal bands and changes in thickness of inner retinal bands.28 Through a different machine learning method, Schmidt-Erfurth et al29 used retinal imaging, demographic, and genetic data to generate and validate a predictive model for assessing risk of conversion from iAMD to CNV and GA in fellow eyes of patients with neovascular AMD that participated in the HARBOR clinical trial of intravitreal ranibizumab. Of 495 eyes analyzed, 45 (9.09%) converted to GA within 2 years. Both the A2A and HARBOR cohorts were classified as iAMD, but all eyes in the A2A cohort had large drusen. The performance characteristics of the predictive model for GA was 0.8, comparable to our random forest model (AUC of 0.80, sensitivity 0.72 and specificity 0.78). The features identified to be most important for conversion to GA were centered on NS retinal features (outer retinal thickness, HRF, and drusen area) and age. Interestingly, these features displayed distinct patterns from those predicting progression to neovascular AMD.29 In contrast with the prior work, ours is the first machine learning analysis that determined short-term (1-year and 2-year) SD-OCT predictors of GA and visual loss in iAMD. Our findings on retinal thicknesses predicting GA onset at 1 or 2 years are in line with the work of de Sisternes et al27 and Schmidt-Erfurth et al,29 whereas SDD appears to be a biomarker for short-term vision loss. It is also worth noting that the presence of age and other demographic risk factors were not identified at high levels in classification/decision trees for GA at 1 year or for visual loss at 1 and 2 years, suggesting that age was not as strongly predictive of these outcomes as for SD-OCT GA at 2 years.
In the current analysis, we found that for the end point GA onset at 1 year, the combination of high HRF axial distance score, NS retinal volume < 90th percentile, high RPEDC abnormal thinning volume, and high RPEDC volume confers a very high (75%) risk of OCT-determined GA at 1 year. In the case of GA onset at 2 years, the presence of photoreceptor outer segment loss over RPE atrophy generated opposing trends in the presence of different risk factors. In combination with high drusen volume, it conferred a high risk of GA onset at 2 years (56% risk; OR, 19.36). The absence of photoreceptor outer segment loss over RPE atrophy, however, was not protective when encountered in combination with age ≥ 80 years and high RPEDC abnormal thinning volume, conferring a 47% risk of OCT-determined GA at 2 years (OR, 13.52). In addition, extremely high “hyperreflective foci axial distance score” ≥ 19 had limited discriminative power and would benefit from external validation, especially that this specific branch of the tree may or may not be reproducible in another dataset. In summary, these results suggest that presence of individual features in isolation is not always predictive of GA, but that the combination of biomarkers is associated with nonexudative AMD progression. These results suggest that the inter-relationship between these variables appears to be most important.
In the classification tree for VA loss ≥ 5 letters during a 2-year interval, contralateral eye VA was associated with a moderate risk and with a high risk in combination with other features (NS retina volume ≥ 15th percentile, SDD, and RPEDC volume ≥ 85th percentile). By contrast, the combination of good VA ≥ 78 ETDRS letters (Snellen equivalent 20/30) and thin NS retina volume (< 15th percentile) resulted in a relatively high (51%) risk of VA loss. This again points to the importance of NS retinal volume in AMD disease progression and confirms that the same SD-OCT features in combination with other OCT biomarkers can be protective or represent a risk factor for GA and VA loss. In the context of more pronounced VA loss (≥ 10 letters) at 1 year, intraretinal cystoid spaces termed “macular edema” in the contralateral eye in combination with lower aspirin use (< 2 per day) or high aspirin use conferred a high risk of VA loss if found in conjunction with different OCT biomarkers. This finding was interesting in the light of a recent study by Chew et al, in which aspirin use was not associated significantly with progression to late AMD in either the AREDS or AREDS2.15 Aspirin use is not uniform in terms of indications, which are usually independent of eye disease, and across various countries. As a result, it may be difficult to generalize findings on aspirin as a risk factor in a US-based population to other worldwide cohorts, as well as in the same population in the future if aspirin indications were to change.
The combination of OCT and visual function (VA) biomarkers can aid in patient selection for clinical trials with specific end points of interest. Although it was not feasible to explore the exact causes of visual loss in the A2A study, there may have been a multitude of causes of visual loss in addition to GA. OCT studies showed that loss of photoreceptors and localized microperimetry changes precedes frank central GA.30 Even if the current quantitative measurements were determined using high-density scans from investigational Bioptigen SD-OCT units in the A2A study, the general trends shown in the classification trees provided can be used to guide patient selection. For example, if a 1-year study of iAMD is planned with a primary outcome of GA development in this short time period, the most suitable candidates would be patients with the following combination of features on OCT: prominent HRF, thin NS retina, and high drusen complex volume or marked thinning of the RPE-drusen complex representing preatrophic changes.
Although most classification trees generated in this work were complex, some findings are easier to translate into a clinical trial design. For example, in the classification tree for OCT-determined GA at 2 years, photoreceptor loss over RPE atrophy had a 34% risk (as opposed to the 7% of the original cohort). This is an example of a result with potential relevance for patients and regulatory agencies because these findings would inform clinical trial design through focused patient selection. The risk of GA development, as computed by the machine learning algorithms, can be used for power calculations to determine the sample sizes of iAMD patients for the planned clinical trial. A reading center will need to be involved in subject selection to advise on the qualitative presence or absence of SD-OCT characteristics (e.g., HRF, photoreceptor outer segment loss, RPE atrophy, thin choroid, macular edema) uncovered by the classification trees. The VA of the contralateral fellow eye will be an additional important consideration for subject inclusion.
Although at the time the AREDS2 trial and A2A substudy were conducted, visual function measurements other than best-corrected VA were not available, more recent evidence suggests that alternative visual function end points may be helpful in further guiding patient selection for clinical trials of iAMD.32, 33, 34 Prevention of progression from intermediate dry AMD to neovascular AMD or GA is key to maintaining functioning vision. In addition, several authors have examined different outcomes or end points in dry AMD, which included patient-centric outcomes and psychophysical tests.31,32,34 This cumulative body of work supports the notion that alternative functional end points together with patient-centric outcomes can be additive to structural outcomes (SD-OCT based) for short-term clinical trials testing the efficacy of therapies in this patient population.
Study Limitations
Our study has several limitations, most notably the sample size of 316 participants, although this is larger than in prior machine learning studies in AMD. The A2A cohort is the largest dataset of iAMD patients with extensive SD-OCT image grading and automated segmentation. A main concern is that some of the OCT features may not be modeled in small sample sizes along some of the branches of the classification trees, leading to overfitting in the model. As a result, caution should be exercised when attempting to generalize results beyond the analyzed population. Another limitation is the narrow cohort definition, which has the potential to limit its applicability in AMD care. However, the A2A study cohort was narrowly defined to address the existing research gaps to best allow defining outcomes for trials that target prevention of atrophy. Of more than 20 recent clinical trials investigating therapeutic options currently in development for non-neovascular AMD, only 1 study relied on the criterion of newly developed atrophy to define its primary outcome.35 Trials that focus on the prevention of progression from iAMD to the more visually significant form (i.e., advanced non-neovascular AMD) are scarce.36 The A2A study, which enrolled a uniquely specific cohort of iAMD, is novel because of its ability to provide classification of iAMD patients according to their risk of atrophy based on a relatively small number of clinical and SD-OCT features.
The current analysis examines the data using the approach used in the Framingham Heart Study, which revolutionized the design of cohorts by optimizing the use of longitudinal data. In this work, we have pooled the same eyes as person-years. This method was theoretically and clinically validated for use in cardiac ischemic disease and generally accepted for use in the literature in chronic diseases that accrue risk factors over time and are irreversible, including diseases of aging such as AMD. Because this is the first time that the methods from the Framingham Heart Study were translated to the field of AMD, external validation will be necessary to provide concrete proof of the independence of eyes used in the analysis.
We were unable to verify the external validity of the findings of this study because of the scarcity of SD-OCT databases with similar follow-up of iAMD. Future work including external validation, more training data, and application of these machine learning algorithms in a large, longitudinal standard of care SD-OCT dataset would aid in validating our results and would enable retinal specialists to make accurate predictions of GA progression and VA loss. Another limitation may be due to potential inaccuracies resulting from the automated segmentation of quantitative SD-OCT features. Although the segmentation and grading methods used here have been previously published and tested for accuracy, a margin of error in baseline and follow-up measurements is expected.
Conclusions
Our machine learning algorithms predict progression from early-intermediate stages of AMD within short-term intervals. The clinical importance of such methods is that they dissect the novel composite end points of GA progression and VA loss, and pave the way for future external validation studies that would allow (1) identification of patients most suitable for clinical trials and (2) tailoring standard-of-care follow-up and clinical decision-making. Our approach is innovative, because it used computational analysis of images in addition to existing clinical data, which would be relatively easy to introduce in the clinical workflow. However, its major limitation is that findings are not externally validated. External validation can be performed in the near future when new datasets become available for longitudinal analysis. For example, the MACUSTAR dataset,21 which is designed with the aim to identify AMD end points, is promising for an enhanced subclassification and prediction of disease progression and will enable earlier treatment and superior clinical outcomes in AMD patients.
Manuscript no. XOPS-D-21-00107.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): E.L.: Consultant – Apellis, Alexion, Galimedix, Gemini Therapeutics, Retrotope, Allegro Ophthalmics, Novartis, Roche/Genentech; Research funding to Duke University – Novartis, Apellis, LumiThera, Roche/Genentech; Research support – National Institutes of Health/National Eye Institute (K23EY026988) and the Veteran’s Affairs Health System (I01 CX002116). The analysis of data from this study was supported by Boehringer Ingelheim. The AREDS2 was supported by the National Eye Institute/National Institutes of Health, Bethesda, Maryland (National Institutes of Health grant no.: 1R01EY023039).
Dr Cynthia Toth, an editor of this journal, and Dr Emily Chew, editor-in-chief of this Journal, were recused from the peer-review process of this article and had no access to information regarding its peer-review.
HUMAN SUBJECTS: Human subjects were included in this study. The study was approved by the Institutional Review Board (IRB) at each of the four clinical sites. Informed written consent was obtained prior to enrollment from each study participant, and the research protocol followed tenets of human research as presented in the Declaration of Helsinki.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Lad, Sleiman, Banks, Hariharan, Herrmann, Dauletbekov, Giani, Chong, Toth
Data collection: Lad, Sleiman, Banks, Hariharan, Clemons, Chew
Analysis and interpretation: Lad, Sleiman, Banks, Hariharan, Clemons, Herrmann, Dauletbekov, Giani, Chong, Chew, Toth
Obtained funding: Lad, Toth
Overall responsibility: Lad, Sleiman, Hariharan, Herrmann, Dauletbekov, Giani, Chong, Toth
Contributor Information
Eleonora M. Lad, Email: nora.lad@duke.edu.
Age-Related Eye Disease Study 2 Ancillary SD-OCT Study Group:
Cynthia A. Toth, Wai Wong, Thomas Huang, G. Baker Hubbard, Sunil Srivastava, Michelle McCall, Katrina Winter, Neeru Sarin, Katherine Hall, Patti McCollum, Linda Curtis, Stefanie Schuman, Stephanie J. Chiu, Sina Farsiu, Vincent Tai, Traci Clemons, and Emily Chew
Supplementary Data
References
- 1.Veerappan M., El-Hage-Sleiman A.M., Tai V., et al. Optical coherence tomography reflective drusen substructures predict progression to geographic atrophy in age-related macular degeneration. Ophthalmology. 2016;123:2554–2570. doi: 10.1016/j.ophtha.2016.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chew E.Y., Clemons T., SanGiovanni J.P., et al. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1) Ophthalmology. 2012;119:2282–2289. doi: 10.1016/j.ophtha.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leuschen J.N., Schuman S.G., Winter K.P., et al. Spectral-domain optical coherence tomography characteristics of intermediate age-related macular degeneration. Ophthalmology. 2013;120:140–150. doi: 10.1016/j.ophtha.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sleiman K., Veerappan M., Winter K.P., et al. Optical coherence tomography predictors of risk for progression to non-neovascular atrophic age-related macular degeneration. Ophthalmology. 2017;124:1764–1777. doi: 10.1016/j.ophtha.2017.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleckenstein M., Schmitz-Valckenberg S., Adrion C., et al. Tracking progression with spectral-domain optical coherence tomography in geographic atrophy caused by age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:3846–3852. doi: 10.1167/iovs.09-4533. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz-Valckenberg S., Fleckenstein M., Göbel A.P., et al. Optical coherence tomography and autofluorescence findings in areas with geographic atrophy due to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:1–6. doi: 10.1167/iovs.10-5619. [DOI] [PubMed] [Google Scholar]
- 7.Wu Z., Luu C.D., Ayton L.N., et al. Optical coherence tomography-defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology. 2014;121:2415–2422. doi: 10.1016/j.ophtha.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 8.Holz F.G., Sadda S.R., Staurenghi G., et al. Imaging protocols in clinical studies in advanced age-related macular degeneration: recommendations from classification of atrophy consensus meetings. Ophthalmology. 2017;124:464–478. doi: 10.1016/j.ophtha.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Sadda S.R., Chakravarthy U., Birch D.G., et al. Clinical endpoints for the study of geographic atrophy secondary to age-related macular degeneration. Retina. 2016;36:1806–1822. doi: 10.1097/IAE.0000000000001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ClinicalTrials.gov AREDS 2 Ancillary Spectral Domain Optical Coherence Tomography Study (A2ASD-OCT): NCT00734487. https://clinicaltrials.gov/ct2/show/study/NCT00734487
- 11.Ferris F.L., Davis M.D., Clemons T.E., et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123:1570–1574. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farsiu S., Chiu S.J., O'Connell R.V., et al. Quantitative classification of eyes with and without intermediate age-related macular degeneration using optical coherence tomography. Ophthalmology. 2014;121:162–172. doi: 10.1016/j.ophtha.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cupples L.A., D'Agostino R.B., Anderson K., Kannel W.B. Comparison of baseline and repeated measure covariate techniques in the Framingham Heart Study. Stat Med. 1988;7:205–222. doi: 10.1002/sim.4780070122. [DOI] [PubMed] [Google Scholar]
- 14.Unal I. Defining an optimal cut-point value in ROC analysis: an alternative approach. Comput Math Methods Med. 2017;2017:3762651. doi: 10.1155/2017/3762651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keenan T.D., Agrón E., Domalpally A., et al. Progression of geographic atrophy in age-related macular degeneration: AREDS2 Report Number 16. Ophthalmology. 2018;125:1913–1928. doi: 10.1016/j.ophtha.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AREDS Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2011;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunness J.S., Rubin G.S., Applegate C.S., et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104:1677–1691. doi: 10.1016/s0161-6420(97)30079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakravarthy U., Bailey C.C., Johnston R.L., et al. Characterizing disease burden and progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:842–849. doi: 10.1016/j.ophtha.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Sunness J.S., Rubin G.S., Broman A., et al. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115:1480–1488. doi: 10.1016/j.ophtha.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ClinicalTrials.gov AMD Ryan Initiative Study (ARIS): NCT03092492. https://clinicaltrials.gov/ct2/show/NCT03092492
- 21.Finger R.P., Schmitz-Valckenberg S., Schmid M., et al. MACUSTAR: Development and clinical validation of functional, structural, and patient-reported endpoints in intermediate age-related macular degeneration. Ophthalmologica. 2019;241:61–72. doi: 10.1159/000491402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folgar F.A., Chow J.H., Farsiu S., et al. Spatial correlation between hyperpigmentary changes on color fundus photography and hyperreflective foci on SD-OCT in intermediate AMD. Invest Ophthalmol Vis Sci. 2012;53:4626–4633. doi: 10.1167/iovs.12-9813. [DOI] [PubMed] [Google Scholar]
- 23.Christenbury J.G., Folgar F.A., O'Connell R.V., et al. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology. 2013;120:1038–1045. doi: 10.1016/j.ophtha.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folgar F.A., Yuan E.L., Sevilla M.B., et al. Drusen volume and retinal pigment epithelium abnormal thinning volume predict 2-year progression of age-related macular degeneration. Ophthalmology. 2016;123:39–50.e1. doi: 10.1016/j.ophtha.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Bogunovic H., Montuoro A., Baratsits M., et al. Machine learning of the progression of intermediate age-related macular degeneration based on OCT imaging. Invest Ophthalmol Vis Sci. 2017;58:141–150. doi: 10.1167/iovs.17-21789. [DOI] [PubMed] [Google Scholar]
- 26.Bogunovic H., Abramoff M., Zhang L., Sonka M. In: Proceedings of the Ophthalmic Medical Image Analysis First International Workshop, OMIA 2014, Held in Conjunction with MICCAI 2014. Chen X., Garvin M.K., Liu J.J., editors. 2014. Prediction of treatment response from retinal OCT in patients with exudative age-related macular degeneration; pp. 129–136. Boston, Massachusetts, September 14. [Google Scholar]
- 27.de Sisternes L., Simon N., Tibshirani R., et al. Quantitative SD-OCT imaging biomarkers as indicators of age-related macular degeneration progression. Invest Ophthalmol Vis Sci. 2014;55:7093–7103. doi: 10.1167/iovs.14-14918. [DOI] [PubMed] [Google Scholar]
- 28.Niu S., de Sisternes L., Chen Q., et al. Fully automated prediction of geographic atrophy growth using quantitative spectral-domain optical coherence tomography biomarkers. Ophthalmology. 2016;123:1737–1750. doi: 10.1016/j.ophtha.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt-Erfurth U., Waldstein S.M., Klimscha S., et al. Prediction of individual disease conversion in early AMD using artificial intelligence. Invest Ophthalmol Vis Sci. 2018;59:3199–3208. doi: 10.1167/iovs.18-24106. [DOI] [PubMed] [Google Scholar]
- 30.Wu Z., Ayton L.N., Luu C.D., Guymer R.H. Microperimetry of nascent geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;56:115–121. doi: 10.1167/iovs.14-15614. [DOI] [PubMed] [Google Scholar]
- 31.Cocce K.J., Stinnett S.S., Luhmann U.F.O., et al. Visual function metrics in early and intermediate dry age-related macular degeneration for use as clinical trial endpoints. Am J Ophthalmol. 2018;189:127–138. doi: 10.1016/j.ajo.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu S.T., Thompson A.C., Stinnett S.S., et al. Longitudinal study of visual function in dry age-related macular degeneration at 12 months. Ophthalmol Retina. 2019;3:637–648. doi: 10.1016/j.oret.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Z., Ayton L.N., Luu C.D., Guymer R.H. Longitudinal changes in microperimetry and low luminance visual acuity in age-related macular degeneration. JAMA Ophthalmol. 2015;133:442–448. doi: 10.1001/jamaophthalmol.2014.5963. [DOI] [PubMed] [Google Scholar]
- 34.Thompson A.C., Luhmann U.F.O., Stinnett S.S., et al. Association of low luminance questionnaire with objective functional measures in early and intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018;59:289–297. doi: 10.1167/iovs.17-22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guymer R.H., Wu Z., Hodgson L.A.B., et al. Subthreshold nanosecond laser intervention in age-related macular degeneration: The LEAD Randomized Controlled Clinical Trial. Ophthalmology. 2019;126:829–838. doi: 10.1016/j.ophtha.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Kim J.B., Lad E.M. Therapeutic options under development for nonneovascular age-related macular degeneration and geographic atrophy. Drugs Aging. 2021;38:17–27. doi: 10.1007/s40266-020-00822-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.