Abstract
Background and purpose:
To prospectively evaluate hippocampal radiation dose volume effects and memory decline following cranial irradiation.
Material and methods:
Effects of hippocampal radiation over a wide range of doses were investigated by combining data from three prospective studies. In one, adults with small cell lung cancer received hippocampal-avoidance prophylactic cranial irradiation. In the other two, adults with glioblastoma multiforme received neural progenitor cell sparing radiation or no sparing with extra dose delivered to subventricular zone. Memory was measured by the Hopkins Verbal Learning Test-Revised Delayed Recall (HVLT-R DR) at 6 months after radiation. Dose–volume histograms were generated and dose–response data were fitted to a nonlinear model.
Results:
Of 60 patients enrolled, 30 were analyzable based on HVLT-R DR testing completion status, baseline HVLT-R DR and intracranial metastasis/recurrence or prior hippocampal resection status. We observed a dose–response of radiation to the hippocampus with regard to decline in HVLT-R DR. D50% of the bilateral hippocampi of 22.1 Gy is associated with 20% risk of decline.
Conclusions:
This prospective study demonstrates an association between hippocampal dose volume effects and memory decline measured by HVLT-R DR over a wide dose range. These data support a potential benefit of hippocampal sparing and encourage continued trial enrollment.
Keywords: Dose response, Memory, Neurocognitive function, Hippocampus, Radiation therapy, Hopkins Verbal Learning Test-Revised
It is well known that decline in neurocognitive function (NCF) is an iatrogenic side effect of brain irradiation [1]. Preclinical and human studies suggest that bilateral or unilateral hippocampal radiation injury may be a key mediator of subsequent NCF decline, most notably in learning and memory [2,3]. The precise pathophysiology of radiation induced neurotoxicity remains to be elucidated; nevertheless, radiation induced damage to neural progenitor cells (NPCs) within the hippocampus may be one of the most compelling [4]. NPCs are exquisitely sensitive to radiation, since doses as low as 2 Gy delivered to human NPCs lead to decreased numbers of cells undergoing neuronal differentiation [5]. Similarly, human studies have demonstrated cognitive deficits after cranial irradiation [6,7]. In light of this, hippocampal-sparing studies have been attempted, most notably in the setting of prophylactic brain irradiation (PCI) in patients with small cell lung cancer [8] and whole-brain radiotherapy (WBRT) for brain metastases [9]. However, these studies largely determined the hippocampal dose constraint by what is technically feasible while maintaining coverage of the normal brain, whereas data regarding the dose–response relationship are still lacking. Gondi et al suggested that a biological equivalent dose in 2-Gy fractions (EQD2) greater than 7.3 Gy applied to 40% of hippocampus was associated with worse NCF [10].
The purpose of this study is to evaluate radiation dose volume effects on memory deficits over a wide range of radiation doses, using data from three prospective trials. These data will provide a framework for future investigations and recommendations for dose reduction to the hippocampus in treating brain metastases and primary brain tumors.
Materials and methods
Patient selection
Patients in the current study were pooled from 3 prospective trials: 21 patients from a phase II trial of hippocampal-sparing PCI for limited-stage small cell lung cancer (SCLC) (HA-PCI group [8]), 30 from a prospective trial of NPC sparing radiation therapy plus temozolomide for newly diagnosed glioblastoma multiforme (GBM) (NPC-GBM group, NCT01478854, accrual complete and manuscript in preparation [11]), and 9 from an ongoing randomized phase II study of subventricular zone (SVZ) irradiation plus concurrent and adjuvant temozolomide in newly diagnosed GBM (SVZ-GBM group, NCT02177578). The study was approved by the Johns Hopkins University Institutional Review Board. The study was performed in accordance with the Declaration of Helsinki. Informed consent was obtained from patients involved in the study.
In the SVZ-GBM group, patients were randomized at a 3:1 ratio to the SVZ irradiation group and NPC sparing group. Eligibility criteria for the HA-PCI group can be found in [8]. The primary inclusion criteria for patients for the latter two GBM trials had newly diagnosed, histologically confirmed GBM, age ≥18 years with KPS >60% and no prior brain radiation, with start of radiation therapy within 12 weeks of biopsy or surgery. In all 3 groups, exclusion criteria for the analyses presented here include failure to complete baseline or follow-up NCF testing (6-month and 12-month), baseline HVLT-R DR (Hopkins Verbal Learning Test-Revised Delayed Recall) score <3, intracranial metastasis/recurrence before NCF testing and prior resection of hippocampus at the time of diagnosis. Among the 41 patients who completed baseline and follow-up NCF testing, 1 (5.2%) in the HA-PCI cohort, 3 (17.6%) in the GBM-NPC cohort and 0 in the GBM-SVZ cohort had intracranial progression before the time of follow-up NCF testing and were therefore excluded from the analysis. None of the patients included in the analysis had progressive disease, received re-irradiation or Avastin chemotherapy at the time of or prior to follow-up that included HVLT-R DR testing. Patients with gross tumor involving the hippocampus were excluded.
Radiation simulation, treatment planning, and procedure
Radiation CT simulation and MRI scan were performed as previously described in [8]. The hippocampus and hippocampus avoidance structure (defined as the hippocampus plus 5-mm radial expansion) were contoured according to the Radiation Therapy Oncology Group atlas. An example is shown in Fig. 1. For all included studies, final delineation of the hippocampus was verified by a single physician (KJR) before the commencement of treatment to ensure consistency. In the HA-PCI group, patients were treated to a total dose of 25 Gy in 10 fractions, administered 5 days per week. An intensity modulated RT plan was generated in which the mean dose to the hippocampus was <8 Gy and at least 90% of the whole brain received 90% of the prescription. In the two GBM groups, patients were initially treated to 46 Gy in 23 fractions, with subsequent cone-down boost in 7 fractions, yielding a total dose of 60 Gy with a once-daily fractionation schedule of 2 Gy per fraction.
Fig. 1.
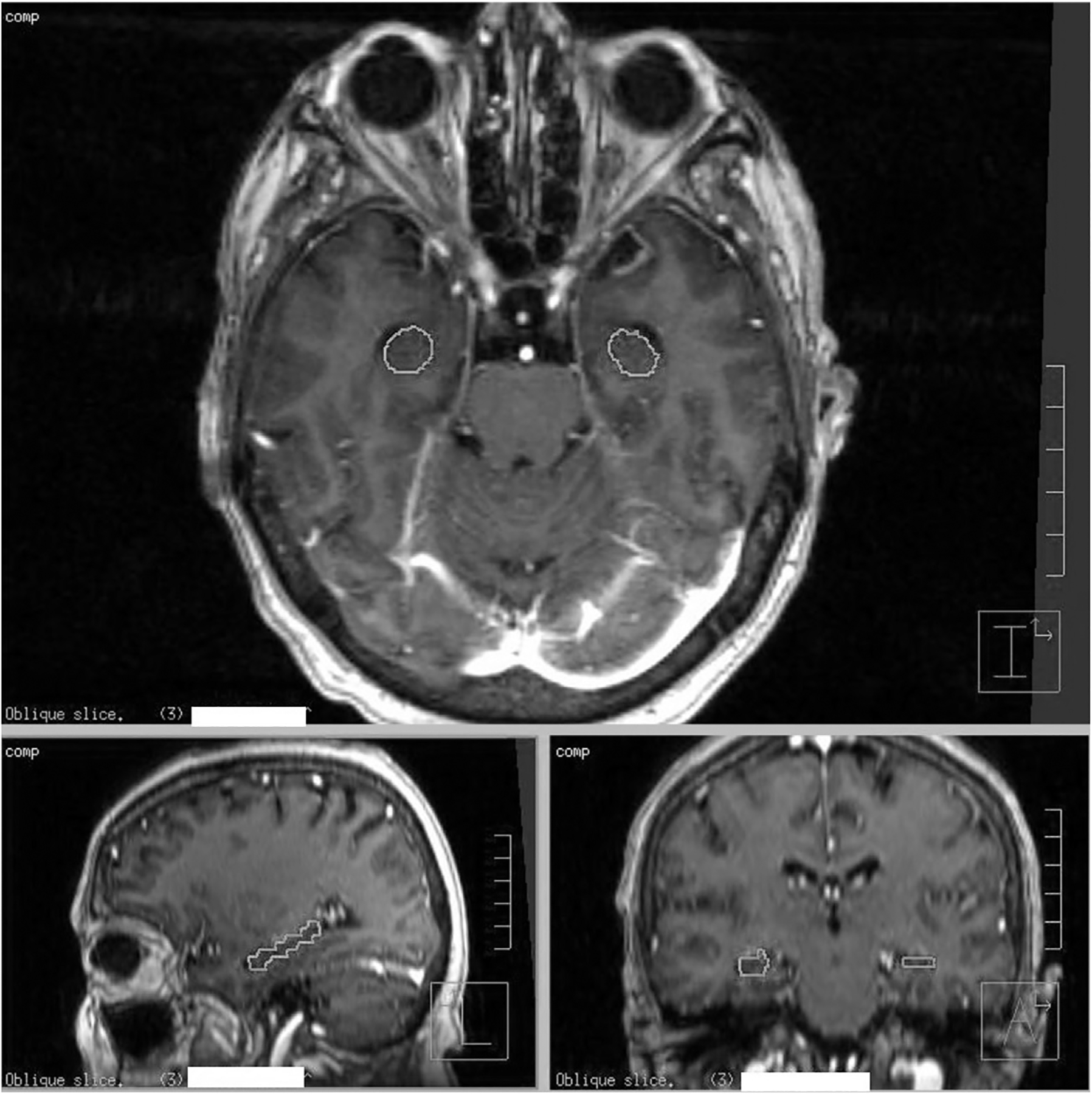
Representative contours of the hippocampus on an axial (top image), sagittal (bottom left) and coronal reconstructions (bottom right) of T1-weighted MRI with postgadolinium contrast. The T1 postgadolinium MRIs were fused to the RT-planning CT scan to allow calculation of the doses to contoured structures. Hippocampus was outlined.
In NPC-GBM and the NPC sparing arm of SVZ-GBM group, a treatment plan was generated which aimed to limit radiation dose to the NPC-containing niches as much as possible without compromising coverage of the planning target volume. The NPC-containing niche was defined as a 5 mm region adjacent to the lateral wall of the lateral ventricle and the entire hippocampus. In the SVZ irradiation arm of the SVZ-GBM group, relatively high doses of radiation were delivered to SVZ and resulting in higher doses to the adjacent hippocampus. In all groups, daily cone-beam CT guidance was used. All patients who initiated protocol treatment were followed per protocol.
Dose-volume histogram (DVH) analysis of hippocampus
DVHs were generated for the left and right hippocampus individually and for the composite bilateral hippocampi. Doses were converted to biologically equivalent doses in 2-Gy fractions (EQD2) assuming an α/β ratio of 2 Gy. EQD2 to deciles (D10% to D100%), and the maximum EQD2 (Dmax) of individual and combined hippocampal volumes were determined and tabulated. The dose-volume data were loaded into the DVH Evaluator software tool [12] and an exponential form of a logistic model was used to generate the dose–response curve:
NTCP is normal tissue complication probability, Dv is the x-axis dose parameter corresponding to volume V, TD50 (V) is the 50% tolerance dose for V, and γ50 (V) is the slope parameter at 50% tolerance dose for V.
Neurocognitive instruments
Participants completed standardized batteries of cognitive tests at baseline and at 6- and 12-month follow up. Test batteries differed slightly across studies. At baseline, estimated pre-morbid intellect was determined via the Hopkins Adult Reading Test [13] in HA-PCI and SVZ-GBM participants. Global cognitive functioning was assessed at baseline via the Mini Mental Status Exam (MMSE) [14] in HA-PCI and NPC-GBM participants. All study participants completed the HVLT-R, a well-validated test of verbal learning and memory [15], as well as Trail Making Test (Part A &B) and Controlled Oral Word Association (COWA) test. However, analysis was restricted to HVLT-R DR as it is the primary end point of multiple hippocampal-sparing radiation trials ([8,9], NCT02635009 and NCT02360215). Delayed recall scores range from 0 to 12, with higher scores reflecting better memory performance.
Statistical methods
The primary endpoint was memory decline, measured by HVLT-R DR at baseline and 6 months after completion of radiation therapy. The Reliable Change Index (RCI) was used to measure meaningful change between baseline and 6 months for HVLT-R DR. The RCI is derived from the standard error of measurement (SEM). SEM, The standard error of the difference (SEdiff) and RCI were calculated as previously described in [8]. For HVLT-R DR, RCI criteria is met if the raw score change is greater than or equal to 3. Therefore a reduction in HVLT-R DR score of 3 or more at 6-month follow-up is considered clinically meaningful memory decline. Multivariate analysis using a linear regression model was performed on the change in HVLT-R DR to evaluate its relationship with age, baseline HVLT-R DR scores and dosimetric variables (D100%, D50% and Dmax). The number of variables was limited by the sample size and number of events. Statistical significance of a predictor was based on a two-sided test of the coefficient with p < 0.05.
Results
Between December 2011 and January 2016, 21, 30 and 9 patients (total = 60) were accrued in the HA-PCI, NPC-GBM and SVZ-GBM studies, respectively. Of 60 patients enrolled, 37 completed both baseline and 6-month HVLT-R DR testing. Four additional patients that did not complete 6-month testing completed 12-month testing. After excluding 7 patients who had baseline HVLT-R DR score <3, 4 who had intracranial metastasis/recurrence before the time of testing, 1 who had gross tumor involvement with resection of the right hippocampus, 30 patients were analyzable. Participant characteristics are summarized in Table 1. There is a wide range of dose to the hippocampus (D50% range 3.9–60.5 Gy) among 3 groups, which is summarized in Table 2. In general, patients in the SVZ-GBM group received the highest dose of radiation to the hippocampus secondary to intentional SVZ irradiation and the anatomical proximity between SVZ and the hippocampus. Participants in the NPC-GBM group received the second highest dose to hippocampus due to tumor proximity to the hippocampus but attempted sparing of the NPC regions (hippocampus and SVZ). Patients with SCLC receiving HA-PCI consistently had the lowest dose of radiation to the hippocampus. Examples of simulation CT with prescription isodose lines are shown in Fig. 2. Two-year OS was 75% (95% CI, 58–97%) for the HA-PCI cohort and OS for the other two ongoing GBM studies will be reported in future publications.
Table 1.
Patient demographics and baseline characteristics (n=30).
| Characteristics | HA-PCI (n = 16) | NPC-GBM (n = 11) | SVZ-GBM (n = 3) | |||
|---|---|---|---|---|---|---|
| No. | % (range) | No. | % (range) | No. | % (range) | |
| Age, years | ||||||
| Median | 59 | 50 | 57 | |||
| Range | 48–76 | 42–77 | 57–61 | |||
| Gender | ||||||
| Male | 6 | 37.5% | 7 | 63.6% | 3 | 100% |
| Female | 10 | 62.5% | 4 | 36.4% | 0 | 0% |
| Race | ||||||
| White | 15 | 93.8% | 10 | 90.9% | 1 | 33.3% |
| Black or African American | 0 | 0% | 1 | 9.1% | 0 | 0% |
| Other | 1 | 6.2% | 0 | 0% | 2 | 66.7% |
| Marital status | ||||||
| Married | 9 | 56.2% | 10 | 83.3% | 3 | 100% |
| Single | 3 | 18.8% | 2 | 16.7% | 0 | 0% |
| Divorced | 3 | 18.8% | 0 | 0% | 0 | 0% |
| Others | 1 | 6.2% | 0 | 0% | 0 | 0% |
| Estimated premorbid IQ | 105.9 | – | 103.0 | |||
| Baseline MMSE | 28.3 | (26–30) | 27.0 | (19–30) | – | – |
| Baseline KPS | 90.0 | 88.3 | 80.0 | |||
| Education, years | 13.1 | (8–19) | 14.2 | (12–16) | 17.0 | (12–22) |
Table 2.
Hippocampal dosimetric parameters of three cohorts.
| HA-PCI (n = 16) | NPC-GBM (n = 11) | SVZ-GBM (n = 3) | |
|---|---|---|---|
| D100% (Gy) | |||
| Mean | 4.2 | 12.0 | 44.7 |
| Range | 3.4–4.8 | 1.0–13.6 | 43.5–45.8 |
| Dmax (Gy) | |||
| Mean | 7.6 | 42.8 | 61.7 |
| Range | 5.5–9.4 | 36.8–64.7 | 60.9–62.2 |
| D50% (Gy) | |||
| Mean | 5.1 | 23.6 | 54.5 |
| Range | 3.9–5.6 | 2.1–61.1 | 50.7–60.5 |
| D1cc (Gy) | |||
| Mean | 5.5 | 37.5 | 58.0 |
| Range | 4.1–6.2 | 14.7–63.5 | 56.3–61.0 |
| D0.5cc (Gy) | |||
| Mean | 5.8 | 40.9 | 60.3 |
| Range | 4.4–6.7 | 23.2–64.1 | 59.3–61.6 |
All doses were expressed in EQD2: biologically equivalent doses in 2-Gy fractions assuming an α/β ratio of 2 Gy. Both hippocampi were included in the analysis.
Fig. 2.
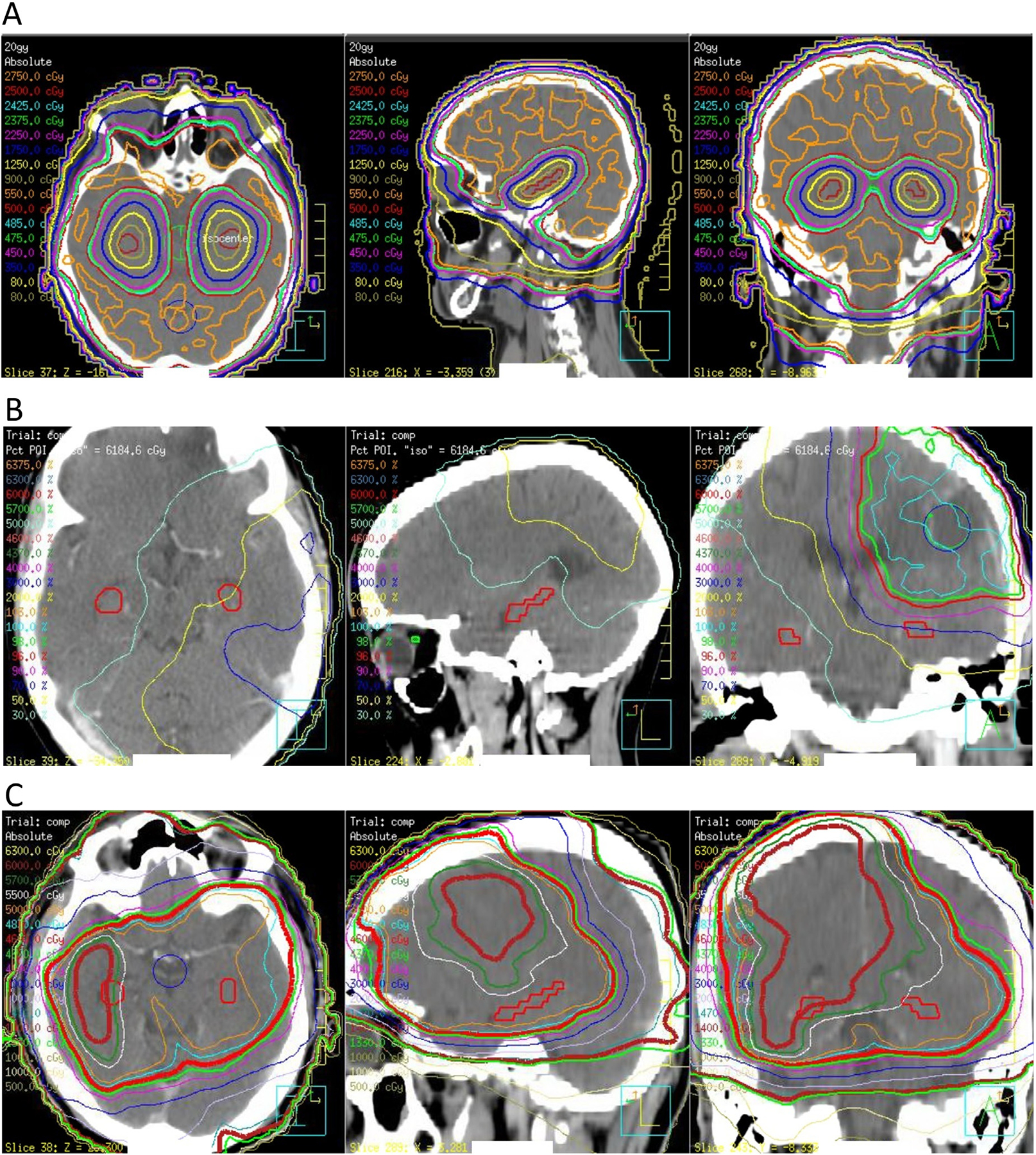
Representative simulation CT images with prescription isodose lines in HA-PCI (A), NPC-GBM (B) and SVZ-GBM (C) cohorts. Hippocampus was outlined in red.
At the time of follow-up, neuro-psychological evaluation decline in HVLT-R DR was noted in 6 patients (20%): 2 (12.5%) in the HA-PCI group, 3 (27.3%) in the NPC-GBM group and 1 (33.3%) in the SVZ-GBM group. Among all participants, the mean HVLT-R DR decline was −0.06 in the HA-PCI group, 0.18 in the NPC-GBM group and 1.67 in the SVZ-GBM group. Predictive relationships between decline in HVLT-R DR and hippocampal dosimetric parameters were studied in detail for the left and right hippocampus individually and for the bilateral hippocampi as a composite structure (Fig. 3).
Fig. 3.
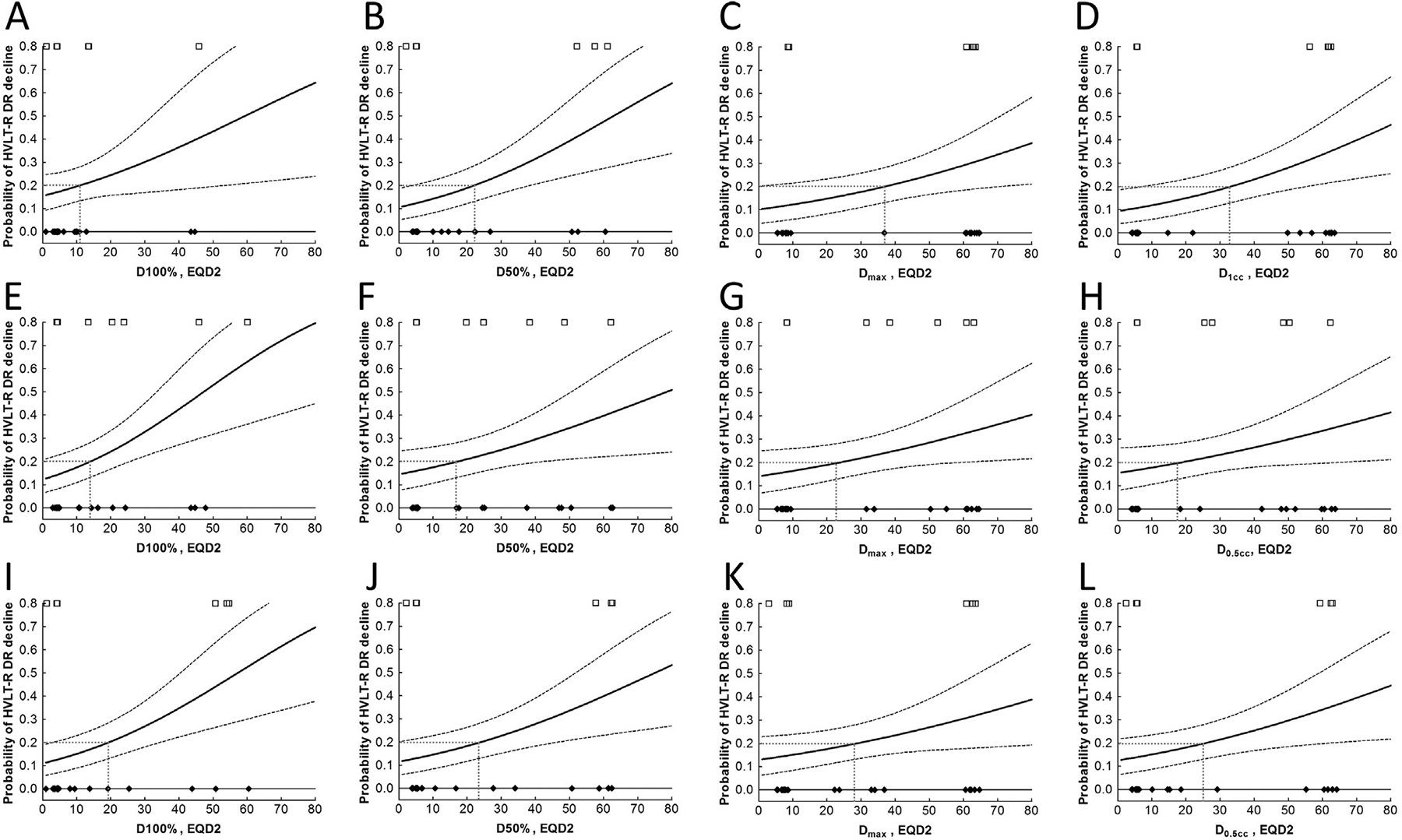
Dose-response relationship between EQD2 to various volumes of bilateral hippocampi (A–D), left hippocampus (E–H) and right hippocampus (I–L) and decline of HVLT-R DR at 6 months. Cases with HVLT-R DR decline were plotted as open squares and cases without HVLT-R DR decline as filled diamonds, the logistic model as the solid line, the 20% risk levels as dotted lines, and the 68% confidence interval as dashed lines.
A dose response was observed for all Dv from D100% (Dmin) to D0cc (Dmax), as well as for all equivalent uniform dose (EUD) [16,17] from n = ±0.01 to n = ±100. D100% to the bilateral hippocampi leading to a 20% probability of HVLT-R DR decline (tolerated dose at 20% risk, TD20) was estimated to be 10.9 Gy; dose leading to a 50% probability of HVLT-R DR decline (TD50) was 59.3 Gy. The slope parameter (γ50) of the dose–response curve in the logistic normal-tissue complication probability (NTCP) model without volume effect was estimated to be 0.42. TD20 and TD50 for D50% to bilateral hippocampi were 22.1 Gy and 62.9 Gy, respectively. TD20 and TD50 for Dmax to bilateral hippocampi were 37.0 Gy and 101.4 Gy, respectively (Table 3). In addition, in the two GBM cohorts (NPC-GBM and SVZ-GBM), Dmax was significantly associated with change in HVLT-R DR score in a linear regression model (p = 0.032 in a univariate analysis and p = 0.001 in a multivariate analysis adjusting for age and baseline HVLT-R DR scores). Furthermore, left and right half hippocampus showed similar dose–response relationship compared to the combined hippocampi.
Table 3.
Dose-response parameters for the logistic model.
| Group | Volume | Slope (γ50) | EQD2 | |
|---|---|---|---|---|
| TD20 (Gy) | TD50 (Gy) | |||
| Both hippocampi | 100% | 0.42 | 10.9 | 59.3 |
| 50% | 0.53 | 22.1 | 62.9 | |
| 0 | 0.55 | 37.0 | 101.4 | |
| 1 cc | 0.57 | 33.3 | 85.5 | |
| Left hippocampus | 100% | 0.49 | 14.0 | 47.3 |
| 50% | 0.44 | 17.1 | 78.4 | |
| 0 | 0.45 | 23.7 | 101.7 | |
| 0.5 cc | 0.42 | 18.4 | 100.4 | |
| Right hippocampus | 100% | 0.52 | 19.3 | 57.2 |
| 50% | 0.51 | 24.1 | 75.2 | |
| 0 | 0.48 | 28.8 | 105.1 | |
| 0.5 cc | 0.49 | 25.9 | 90.0 | |
TDX: tolerated dose (EQD2) resulting in X% risk of HVLT-R DR decline in 6 months.
The slope parameter (γ50) of the dose–response curve in the logistic normal-tissue complication probability (NTCP) model without volume effect is calculated at TD50.
Discussion
In recent years, there has been growing interest in hippocampal sparing radiation therapy with accumulating evidence that radiation induced damage to neural progenitor cells (NPCs) within the hippocampus may play a key role in the NCF decline after brain irradiation. Hippocampal sparing radiation has been studied in the setting of PCI in patients with SCLC [8], WBRT for brain metastases [9], adult glioma [11,18] and pediatric medulloblastoma [19].
The safety and efficacy of this approach is currently being investigated in the context of a randomized controlled trial in patients receiving PCI for both limited and extensive stage SCLC (NRG-CC003, NCT02635009) and in patients receiving WBRT for brain metastases (NRG-CC001, NCT02360215). These trials will be essential to more fully illustrate the effect of hippocampal radiation on cognitive function and the potential benefit in hippocampal avoidance. Although preliminary investigations suggest that it is likely safe and does not significantly increase the risk of treatment failure [21–23], the approach remains investigational and should not yet be performed outside of the clinical trial setting. Specifically in the context of glioblastoma, there have been reports that the tumor tends to recur near neural stem cell regions including the subgranular zone (SGZ) of the hippocampus as well as the SVZ [24] and that increased radiation dose to the SVZ may improve overall and/or progression free survival [25–27]. Furthermore, preliminary data from studies of hippocampal avoidance have demonstrated development of metastases in under-dosed regions of the brain [8,21].
Importantly, the radiation dose constraints utilized in the studies reported to date are not well characterized, likely because of minimal data available to guide it. As a result, current studies aim predominantly to meet constraints determined by technical purposes based on the feasibility of sparing while otherwise maintaining coverage as appropriate for oncologic purposes. For example, in RTOG 0933 trial, the dose to 100% of the hippocampus was limited to 9 Gy, and maximal hippocampal dose to 16 Gy [9]. Nonetheless, detailed dose–volume analyses are vital in guiding the clinician in striking the balance between local tumor control and NCF preservation. There are only a few related studies published to date [10,28,29] and in these studies the ranges of dose to the hippocampus were relatively narrow.
We present one of the first prospective evaluations of the dose–response relationship utilizing HVLT-R DR over a wide range of radiation doses to the hippocampus. We observed a dose–response for both unilateral and combined hippocampi, consistent with pre-clinical evidence suggesting that neurocognitive dysfunction manifests at much lower doses (5–10 Gy) than previously expected [2,30]. Specifically, in our study, D50% of EQD2 = 22.1 Gy to the bilateral hippocampi leads to a 20% probability of HVLT-R DR decline (TD20) with a slope parameter (γ50) of 0.53. Interestingly, the left hippocampus may be more sensitive to radiation than the right leading to HVLT-R DR decline (Table 3). This is consistent with the observation that visuo-spatial memory is predominantly associated with the right whereas verbal or narrative memory with the left [31,32].
Our study has several limitations. First, the number of analyzable patients was limited by those who completed both baseline and follow-up NCF testing. One challenge was the level of compliance; although compliance was high at baseline for all 3 groups and maintained in the HA-PCI group at 6 months (95.0%) and 12 months (73.7%), it dropped to 48.6% and 47.3% at 6 months and 12 months for the GBM groups. This is consistent with other studies in this patient population [33,34] where compliance with testing is inherently limited by the aggressive nature of the disease, poor life expectancy, and the resulting complex psychosocial situation. The current study is hypothesis-generating rather than definitive given relatively low number of patients and number of events. Therefore the results should be interpreted with caution. Nevertheless, the sample size of our study compares favorably to previous dose–response studies, making this manuscript an important contribution to the literature [10,28]. Second, the length of follow-up is limited to 6 months in the majority of patients. While 16 patients completed both 6- and 12-month follow-up, most (n = 12, 75%) were from the HA-PCI study. This is mainly due to the poor median survival in GBM patients. We did not analyze the 12 month time point due to the possibility of skewing toward healthier patients. Nevertheless, our follow-up time is comparable to RTOG 0933, in which data from 4- and 6-month follow-up were analyzed, due to median survival of 6.8 months. Third, our patient population represents a mixture of tumor types, with 16 (53%) patients with SCLC without brain metastasis and 14 (47%) with GBM at the time of diagnosis. This could be potentially confounding due to the inherently different biology of the cancer and chemotherapy regimens. However, it should be noted that even when the analysis was limited to the two GBM cohorts, Dmax correlated significantly with change in HVLT-R DR score in a multivariate analysis. Therefore the dose–response relationship remains when tumor type and chemotherapy regimen were fixed, arguing against the possibility that the dose–response relationship across tumor types was solely a result of difference in the aggressiveness of the cancer and the types of chemotherapy. The anti-tumor effect of radiation in GBM patients may also contribute to NCF improvement [35]. However, this diverse patient population enabled us to study the effect of hippocampal radiation over a wide range of doses.
Nonetheless, this manuscript is a uniquely important contribution to the literature for several reasons. First, we are the first study employing a dose–response model over a wide range of hippocampal radiation doses (D50% range 3.9–60.5 Gy), and all included data are of high quality collected in a prospective manner. This strategy of modeling, if validated in a separate cohort, will potentially allow clinicians to evaluate alternative treatment plans based on acceptable risk levels appropriate for each unique clinical situation to better optimize radiation treatment. In addition, it will also allow clinicians to become more comfortable in devising more aggressive regimens when necessary such as for radio-resistant tumors to improve the effectiveness of treatment. Second, we are the first to use HVLT-R DR as the endpoint. HVLT-R is a measurement tool widely used in adult radiation oncology trials. It has good validity and reliability and is well tolerated by elderly people and applicable across cultures [36,37].
Conclusion
This prospective study demonstrates an association between hippocampal radiation dose volume effects and memory deficits measured by HVLT-R DR over a wide range of doses to the hippocampus. 22.1 Gy of D50% to the bilateral hippocampi led to a 20% probability of memory deficit as measured by HVLT-R DR decline. The exact hippocampal dose constraints should be tailored to each clinical situation based on the unique patient situation and a balance between maximizing oncological control and minimizing neurocognitive impact. The curves presented here may be used for reference to assist with this decision making. In addition, these data may provide a framework for future investigation of dose reduction to hippocampus in the management of brain metastases and primary brain tumors.
Acknowledgement
We would like to acknowledge support for partial statistical analysis from Carol Thompson from the Johns Hopkins University School of Public Health, the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 1UL1TR001079.
Conflict of interest statement
KJR has received research funding from Elekta AB, honorarium for educational activity from AstraZeneca, and consultant activities for Medtronic.
JG designed and holds intellectual property rights to the DVH Evaluator software tool which is an FDA-cleared product in commercial use, and which has been used for this analysis.
All other authors have no conflict of interest to disclose.
Neither the authors nor the Johns Hopkins University receives royalties for the Hopkins Verbal Learning Test (HVLT).
References
- [1].Tallet AV, Azria D, Barlesi F, et al. Neurocognitive function impairment after whole brain radiotherapy for brain metastases: actual assessment. Radiat Oncol (London, England) 2012;7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Raber J, Rola R, LeFevour A, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res 2004;162:39–47. [DOI] [PubMed] [Google Scholar]
- [3].Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res 2000;153:357–70. [DOI] [PubMed] [Google Scholar]
- [4].Wong CS, Van der Kogel AJ. Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Intervent 2004;4:273–84. [DOI] [PubMed] [Google Scholar]
- [5].Acharya MM, Lan ML, Kan VH, et al. Consequences of ionizing radiation-induced damage in human neural stem cells. Free Radic Biol Med 2010;49:1846–55. [DOI] [PubMed] [Google Scholar]
- [6].Redmond KJ, Mahone EM, Horska A. Association between radiation dose to neuronal progenitor cell niches and temporal lobes and performance on neuropsychological testing in children: a prospective study. Neuro-oncology 2013;15:1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wolfson AH, Bae K, Komaki R, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;81:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Redmond KJ, Hales RK, Anderson-Keightly H, et al. Prospective study of hippocampal-sparing prophylactic cranial irradiation in limited-stage small cell lung cancer. Int J Radiat Oncol Biol Phys 2017;98:603–11. [DOI] [PubMed] [Google Scholar]
- [9].Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 2014;32:3810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gondi V, Hermann BP, Mehta MP, Tome WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys 2012;83:e487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Redmond KJ, Ye X, Assadi RK, et al. Neural progenitor cell (NPC) sparing radiation therapy (RT) plus temozolomide (TMZ) in newly diagnosed glioblastoma multiforme (GBM) associated with neurocognitive function but not tumor outcomes: results of a prospective clinical trial. Int J Radiat Oncol Biol Phys 2015;93:S111. [Google Scholar]
- [12].Grimm J, Sahgal A, Soltys SG, et al. Estimated risk level of unified stereotactic body radiation therapy dose tolerance limits for spinal cord. Semin Radiat Oncol 2016;26:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schretlen DJ, Winicki JM, Meyer SM, Testa SM, Pearlson GD, Gordon B. Development, psychometric properties, and validity of the hopkins adult reading test (HART). Clin Neuropsychol 2009;23:926–43. [DOI] [PubMed] [Google Scholar]
- [14].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [15].Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test - Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol 1998;12:43–55. [Google Scholar]
- [16].Mohan R, Mageras GS, Baldwin B, et al. Clinically relevant optimization of 3-D conformal treatments. Med Phys 1992;19:933–44. [DOI] [PubMed] [Google Scholar]
- [17].Niemierko A Reporting and analyzing dose distributions: a concept of equivalent uniform dose. Med Phys 1997;24:103–10. [DOI] [PubMed] [Google Scholar]
- [18].Marsh JC, Godbole R, Diaz AZ, Gielda BT, Turian JV. Sparing of the hippocampus, limbic circuit and neural stem cell compartment during partial brain radiotherapy for glioma: a dosimetric feasibility study. J Med Imag Radiat Oncol 2011;55:442–9. [DOI] [PubMed] [Google Scholar]
- [19].Brodin NP, Munck af Rosenschold P, Blomstrand M, et al. Hippocampal sparing radiotherapy for pediatric medulloblastoma: impact of treatment margins and treatment technique. Neuro-oncology 2014;16:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Marsh JC, Herskovic AM, Gielda BT, et al. Intracranial metastatic disease spares the limbic circuit: a review of 697 metastatic lesions in 107 patients. Int J Radiat Oncol Biol Phys 2010;76:504–12. [DOI] [PubMed] [Google Scholar]
- [21].Gondi V, Tome WA, Marsh J, et al. Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: safety profile for RTOG 0933. Radiother Oncol 2010;95:327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ghia A, Tome WA, Thomas S, et al. Distribution of brain metastases in relation to the hippocampus: implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys 2007;68:971–7. [DOI] [PubMed] [Google Scholar]
- [23].Hong AM, Suo C, Valenzuela M, et al. Low incidence of melanoma brain metastasis in the hippocampus. Radiother Oncol 2014;111:59–62. [DOI] [PubMed] [Google Scholar]
- [24].Chen L, Chaichana KL, Kleinberg L, Ye X, Quinones-Hinojosa A, Redmond K. Glioblastoma recurrence patterns near neural stem cell regions. Radiother Oncol 2015;116:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen L, Guerrero-Cazares H, Ye X, et al. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int J Radiat Oncol Biol Phys 2013;86:616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee P, Eppinga W, Lagerwaard F, et al. Evaluation of high ipsilateral subventricular zone radiation therapy dose in glioblastoma: a pooled analysis. Int J Radiat Oncol Biol Phys 2013;86:609–15. [DOI] [PubMed] [Google Scholar]
- [27].Evers P, Lee PP, DeMarco J, et al. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer 2010;10:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tsai PF, Yang CC, Chuang CC, et al. Hippocampal dosimetry correlates with the change in neurocognitive function after hippocampal sparing during whole brain radiotherapy: a prospective study. Radiat Oncol (London, England) 2015;10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Okoukoni C, McTyre E, Peiffer AM, et al. Hippocampal dosimetry predicts for cancer-related cognitive impairment in patients treated with cranial radiation therapy: dosimetric results of a prospective clinical trial. Int J Radiat Oncol Biol Phys 2016;96:S90–1. [Google Scholar]
- [30].Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Experiment Neurol 2004;188:316–30. [DOI] [PubMed] [Google Scholar]
- [31].Frisk V, Milner B. The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia 1990;28:349–59. [DOI] [PubMed] [Google Scholar]
- [32].Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron 2002;35:625–41. [DOI] [PubMed] [Google Scholar]
- [33].Joly F, Vardy J, Pintilie M, Tannock IF. Quality of life and/or symptom control in randomized clinical trials for patients with advanced cancer. Ann Oncol 2007;18:1935–42. [DOI] [PubMed] [Google Scholar]
- [34].Taphoorn MJ, Stupp R, Coens C, et al. Health-related quality of life in patients with glioblastoma: a randomised controlled trial. Lancet Oncol 2005;6:937–44. [DOI] [PubMed] [Google Scholar]
- [35].Armstrong C, Ruffer J, Corn B, DeVries K, Mollman J. Biphasic patterns of memory deficits following moderate-dose partial-brain irradiation: neuropsychologic outcome and proposed mechanisms. J Clin Oncol 1995;13:2263–71. [DOI] [PubMed] [Google Scholar]
- [36].Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol 1999;13:348–58. [DOI] [PubMed] [Google Scholar]
- [37].Brandt J, Benedict RHB. Hopkins Verbal Learning Test-Revised professional manual. Odessa, FL: Psychological Assessment Resources; 2011. [Google Scholar]


