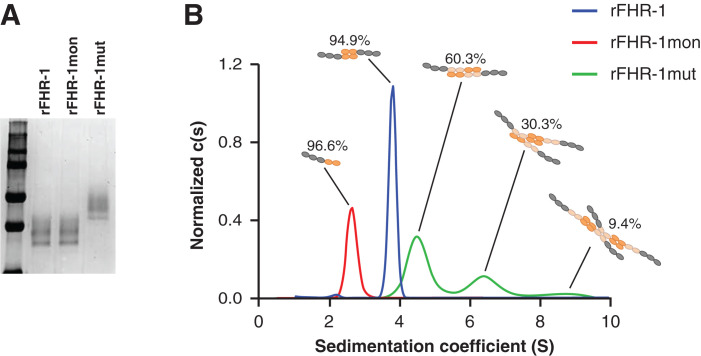
Figure 1.
FHR-1 mutant with duplicated dimerization domains forms multimers. (A) Coomassie-stained SDS-PAGE gel of purified rFHR-1, FHR-1mon, and FHR-1mut. (B) Continuous sedimentation coefficient distribution analysis c(s) of velocity sedimentation experiments of rFHR-1, rFHR-1mut, and rFHR-1mon, performed by analytic ultracentrifugation, revealed single peaks for rFHR-1 and rFHR-1mon with coefficients 3.8S and 2.7S, which are compatible with a dimer and a monomer, respectively. In contrast, rFHR-1mut displayed several peaks at 4.6S, 6.5S, and 8.7S, compatible with dimers, trimers, and tetramers, respectively.