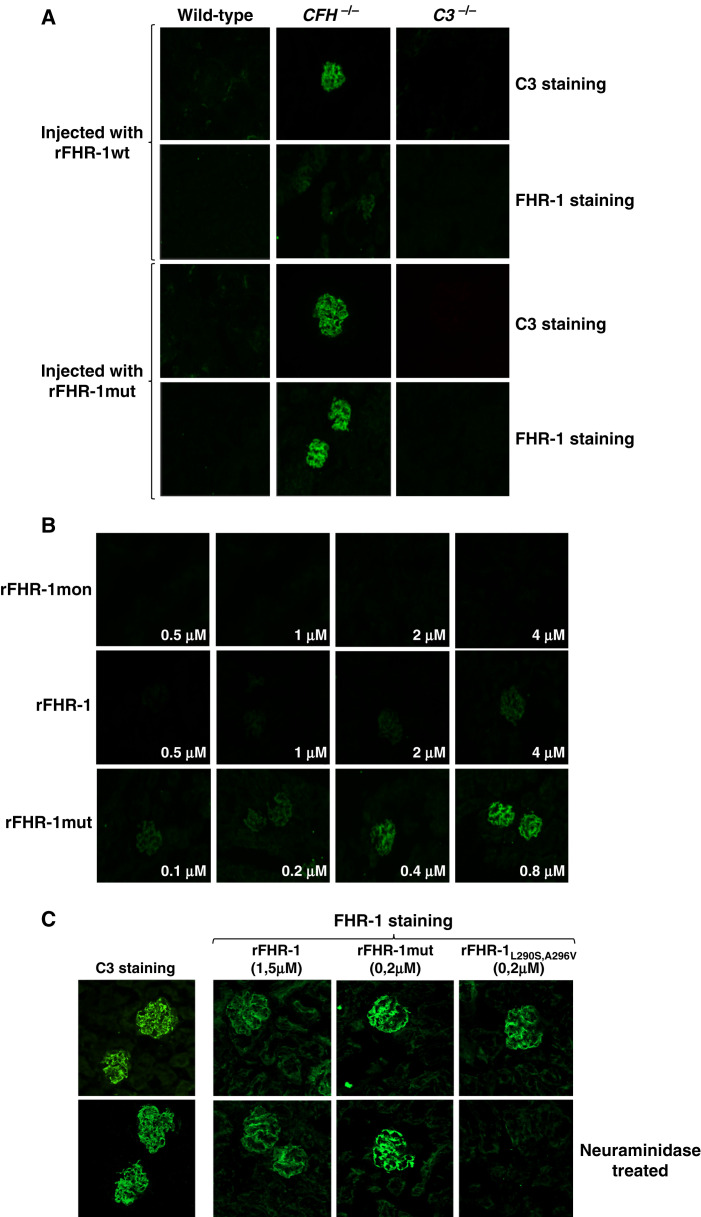
Figure 3.
Binding of FHR-1 to the glomeruli is dependent on C3 deposition and independent of the presence of sialic acid on the surface. (A) Immunofluorescence analysis of renal C3 and FHR-1 proteins in kidney sections of Cfh−/−, wild-type, and C3−/− mice injected with identical amounts of either rFHR-1wt or rFHR-1mut. A significantly higher binding of rFHR-1mut compared with rFHR-1wt was observed in the Cfh−/− mice. This binding was dependent on C3 deposition because it was not observed in wild-type or C3−/− mice, which are negative for C3 staining. (B) Ex vivo, dose-dependent binding of FHR-1 proteins to kidney sections of Cfh−/− animals. Increasing amounts of rFHR-1mon (1–8 µg), rFHR-1 (1–8 µg), and rFHR-1mut (0.25–2 µg) were incubated with kidney sections of Cfh−/−mice, and the binding of these proteins to the surface was detected with the anti-FHR-1 2C6 mAB. (C) Ex vivo binding experiments of FHR-1 proteins to cryostat kidney sections of Cfh−/− mice untreated or treated with neuraminidase. The binding of the rFHR-1wt and rFHR-1mut to the glomeruli is not affected by treatment with neuraminidase. In contrast, binding of the aHUS-associated FHR-1L290S,A296V mutant protein is greatly affected, as previously described.10 This protein was used as a positive control to confirm the removal of sialic acids from the surface. Original magnification, ×40.