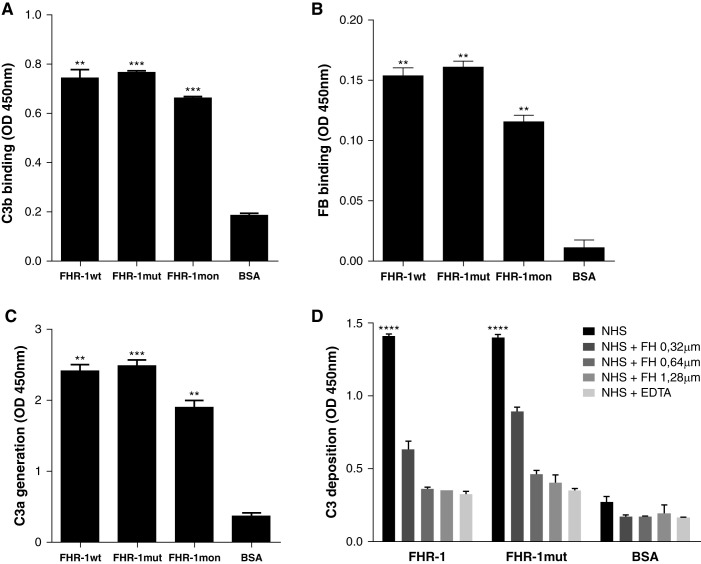
Figure 5.
Surface-bound FHR-1 proteins promote complement activation through the AP by supporting the formation of the C3 convertase. The sequential addition of purified C3b, FB, and FD on a microtiter plate coated with rFHR-1 proteins (wild type, mutant, and monomer) or BSA led to the formation of a C3 convertase only on rFHR-1 proteins, as illustrated by the detection of both (A) C3b and (B) FB. (C) The generation of a functional C3 convertase on FHR-1–coated surfaces is illustrated by the detection of C3a in supernatants after incubating C3 with the C3b-FB complexes previously formed, like in (A and B). (D) Deposition of C3 on microtiter plates coated with either rFHR-1 proteins or BSA and incubated with 10% NHS in the presence of buffer containing either EGTA or EDTA, or supplemented with increasing amounts of FH. Deposition of C3 was detected with the anti-C3 12.17 antibody. In all panels, a representative experiment of a minimum of two independent experiments is shown. Means±SD of triplicates are depicted. Statistical differences between the FHR-1 proteins and the BSA control were calculated. **P<0.01, *** P<0.001, ****P<0.0001, t test.