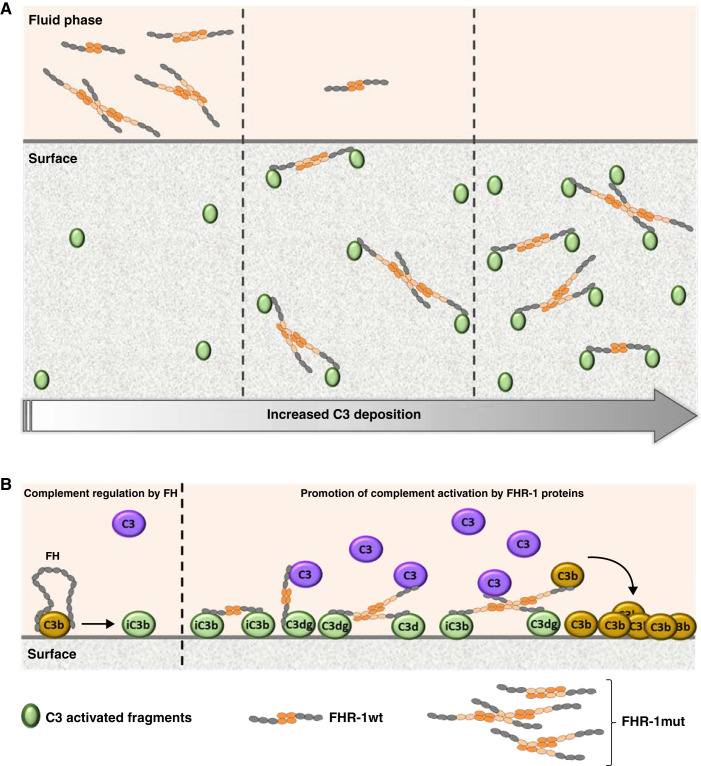
Figure 8.
Proposed mechanistic model for the promotion of complement activation by the C3G-associated FHR-1 mutations with duplicated dimerization domains. A two-step model is proposed on the basis of the main functional differences found between FHR-1wt and FHR-1mut. (A) First, FHR-1mut displays an enhanced avidity to bind C3-activated fragments, which translates into an advantage compared with the wild-type protein to bind to a surface when the density of C3 fragments is lower, as illustrated by the different scenarios with different degrees of C3 deposition. These different scenarios may be due to different time points but may also be related to different surface contexts. (B) Second, FHR-1 proteins bound to a C3-opsonized surface recruit C3 (predominantly) or C3b molecules from the fluid phase, increasing the concentration of potential components to form the AP C3 convertase, promoting an excessive complement activation and deposition of additional C3-activated fragments that overcome FH regulation. In this context, the multimeric forms of the mutant protein may also have an advantage compared with the wild-type protein to simultaneously bind to the C3-opsonized surface and recruit the C3 molecules from the fluid phase.