Abstract
Fluorescent Si-rhodamines were modified to enable complexation with the Re(I)- and 99mTc(I)-tricarbonyl core. The corresponding complexes exhibit suitable properties as bimodal imaging probes for SPECT- and optical imaging in vitro. Importantly, the novel in- aqueous-solution-stable, functionalized Si-rhodamines retain favourable optical properties after complexation (QY=0.09, λabs=654 nm, λem=669 nm in PBS) and show promising near-infrared optical properties for potential in vivo applications enabling bimodal scintigraphic imaging and optical imaging, e.g. used in radio- and fluorescence-guided tumor resection.
Table of contents entry
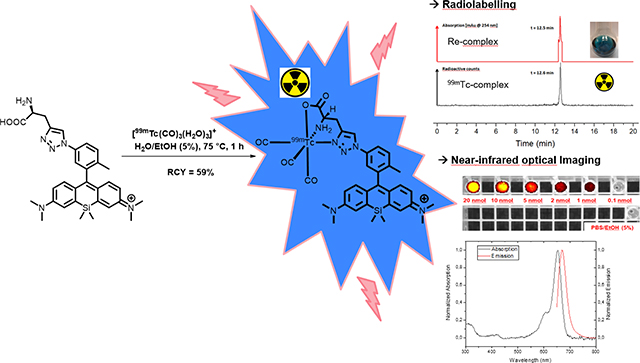
“The first technetium-99m tricarbonyl core labelled fluorescent Si-rhodamine and its rhenium analogue for bimodal SPECT- and near-infrared fluorescence imaging is presented”
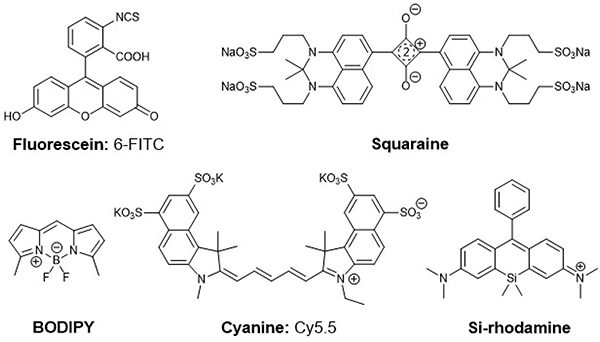
Organic fluorophores are in high demand as imaging probes in biomedical research.1, 2 Optical dyes are generally used for cellular staining, biomolecule characterisation or the visualization of cellular and molecular processes in vivo.3–5 The most extensively studied organic fluorescent dyes for biological applications are fluorescein, squaraine-, BODIPY- and cyanine-dyes (figure 1).1, 6 At this time, the FDA approved fluorophores indocyanine green (ICG) and 5-aminolevulinic acid (5-ALA) are the most established compounds for intraoperative surgical interventions.7 These organic dyes possess high molar extinction coefficients, large stokes shifts and high quantum yields.1 However, in exception of some fluorophores with reduced molecular weight, most of the dyes e.g. members of the FITC family, typically show limited optical properties not compatible with in vivo applications due to their absorbance and emission in the visible part of the spectrum since their absorbance and emission maxima interfere with the natural absorbance of tissue and blood.6, 8–11 In contrast, NIR dyes do not overlap with the absorbance of water and haemoglobin, and provide low phototoxicity in cells and tissue.8 These advantages qualify near-infrared (NIR) dyes particularly powerful for optical imaging. The indocyanine (Cy) dye family provides a suitable alternative to bright, short wave optical probes, as emission properties can be adjusted to NIR compatibility by chain extension. However, indocyanines exhibit decreased solubility in aqueous solution and are sensitive to photobleaching, which can pose limitations to their application.6 Clearly, there is a need for optical probes with small molecular footprint, necessary hydrophilicity for biological applications and strongly red-shifted absorbance and emission. In 2011 Nagano et al. developed the near-infrared silicon rhodamines (SiR) with a maximal absorption wavelength of λabs= 646nm and an emission wavelength of λem= 667nm (figure 1).5, 12, 13 Due to their optical properties in the near-infrared wavelength region the SiR dyes exhibit biocompatible characteristics: enhanced photostability and water solubility, reduced autofluorescence, decreased light scattering- and photobleaching properties.5, 12, 13
Figure 1:
Overview of prominent organic fluorescent dyes and chemical structures of fluorescein, squaraine-, BODIPY-, cyanine and silicon-rhodamine dyes.
Molecular imaging methods with optical imaging probes are used extensively for the visualization of cellular processes.3–5 Although this method is considered to also provide high spatial resolution in the μm-scope and high sensitivity in vivo, the lack of deep tissue penetration can be compensated by use of complementary imaging techniques.14 Nuclear imaging methods such as positron emission tomography (PET) and single photon emission computed tomography (SPECT)-imaging are suitable for this purpose due to their tissue penetration characteristics and high sensitivity suitable for whole body imaging.15 Conclusively the combination of optical and nuclear imaging methods provides synergistic effects, resulting in high spatial resolution and high tissue penetration from the whole body to the subcellular level.15–17 Related to this topic several small molecule based organic optical- and nuclear imaging probes have been evaluated and reported.18 However fluorescent dyes including radiolabelled and functionalized BODIPYs, cyanine or fluorescein-conjugated imaging probes often show a low accumulation in tumors likely because of their enhanced lipophilicity and high enrichment in kidney or liver.17, 19–23 For this reason there is a need of small molecule based water-soluble near-infrared absorbing and emitting probes for bimodal imaging and subsequent fluorescence-guided surgery.
Here, we used the click-to-chelate concept from Mindt et al. to combine the single-photon emitter technetium-99m (physical half-life of 6.02 hours and gamma energy of 141 keV) and the near-infrared fluorophore silicon-rhodamine in one small molecule for its further development for bimodal scintigraphic and optical imaging, e.g. used in radio- and fluorescence-guided intraoperative tumor resection.24, 25
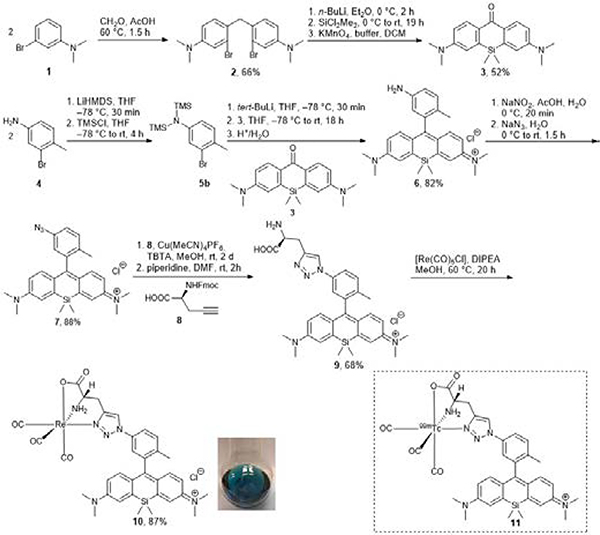
In order to incorporate 99mTc(I) onto the SiR dye, we used a tridentate donor for the coordination of the 99mTc-tricarbonyl core. The synthesis of the non-radioactive rhenium surrogate complex 10 and the technetium-99m complex 11 was achieved from 3-bromo-N,N-dimethylaniline (1) within seven steps (Scheme 1).
Scheme 1:
Reaction pathway for the synthesis of the rhenium Si-rhodamine 10 and the structure of the technetium-99m radiolabelled Si-rhodamine 11.
An azido-functionalized Si-rhodamine 7 was converted by the copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) into the corresponding 1,4-substituted 1,2,3-triazole and subsequently complexed with rhenium(I) as a non-radioactive surrogate for chemical characterization.
To obtain dye 7, we carried out the synthesis following the modification of published procedures: the commercially available 3-bromo-N,N-dimethylaniline (1) was converted using the Blanc reaction to compound 2.26 Using a modified procedure from Bertozzi et al., silicon-xanthone 3 was synthesized with a yield of 52%.27 Xanthone 3 was converted by nucleophilic addition with trimethylsilyl-(TMS) protected anilines 5 to the amine-functionalized SiR 6.28
Subsequently, SiR 6 was converted to the corresponding azide 7 with sodium azide. Formation of the corresponding 1,2,3-triazole-functionalized Si-rhodamine and subsequent complexation with Re(I) and Tc(I) were inspired by the click-to-chelate concept of Mindt et al.24, 29 Various attempts at the copper(I)-catalyzed alkyne-azide click-reaction (CuAAC) conditions were carried out using Fmoc-L-propargylglycine 8 to obtain the Fmoc-protected 1,4-disubstituted 1,2,3-triazole.
Reactions using conventional CuAAC conditions with copper(II) sulfate and sodium ascorbate were not successful; instead, we used the copper(I) source Cu(MeCN)4PF6 with tris((1-benzyl-4-triazolyl)methyl)amine (TBTA) in methanol.28 This method gave the Fmoc-protected product in 87% yield. Finally, the Fmoc-group was removed using base-catalyzed deprotection in DMF to obtain 9 with a yield of 68%. The usage of unprotected L-propargylglycine as the alkyne source for the click reaction gave triazole 9 in an 18% yield only, possibly due to its diminished solubility in methanol. As Rhenium(I) and technetium(I)-complexes have often similar chemical properties, the rhenium complex 10 is considered as a suitable non-radioactive surrogate for the chemical characterization of the corresponding radioactive technetium-complex 11.30 Rhenium complex 10 was synthesized by complexation with pentacarbonylchlororhenium(I) with a yield of 87% after HPLC purification. The analytical HPLC showed a double peak signal which probably indicates two different rotameric structures of 10 (ESI-figure 47) due to the restricted rotation of the aryl-aryl axis from the Si-rhodamine backbone. The presence of rotamers is further evidenced by the HR-ESI-MS data from the double peak signal in HPLC, where one single mass of [M]+ with the correct isotopic pattern expected for 185/187Re is observed. However only one set of chemical shift data was observed via NMR for this double signal at room temperature (ESI-figure 13/14).
A comparison of the proton 1H-NMR data of the ligand 9 and the corresponding rhenium complex 10 indicates a downshift of signals following complexation, correlating well with previous published results (ESI-table 3).24, 29
This is further evidenced by the IR-data of complex 10, which shows the well-defined and characteristic stretching frequency of CO bound to the rhenium core at = 2022 and 1889 cm−1 (ESI-figure 44). The optical properties of all Si-rhodamine derivatives were determined in various solvents and are shown in Table 1. With exception of 6 all other compounds show characteristic absorption- and emission properties in the NIR-region between 650 nm and 675 nm. The molar absorptivity of the SiRs in methanol is somewhat higher than in aqueous solution, and slightly decreases as the degree of substitution of the Si-rhodamines increases. The lowest molar absorption coefficients were obtained for the rhenium complex 10, presumably by competing absorbance and emission from a metal-to-ligand charge-transfer (MLCT) band of the rhenium(I) complex; however, no further efforts were made to elucidate the source of observed change in molar absorptivity; comparable reduction of molar absorption coefficients following rhenium complexation with different fluorophores has been reported by others elsewhere.31–33
Table 1.
Optical properties of the synthesized Si-rhodamines and the Re-complex 10. Excitation was performed at λexc= 650 nm.
| Solvent | λabs, max | λem | εmax | ΦF | |
|---|---|---|---|---|---|
| 6 | MeOH | 653 nm | - | 91900 M−1cm−1 | - |
| PBS (pH = 7.4) | 651 nm | - | 77300 M−1cm−1 | - | |
| 7 | MeOH | 651 nm | 670 nm | 156500 M−1cm−1 | 0.18 |
| H2O/EtOH (5%) | 651 nm | 670 nm | 123700 M−1cm−1 | 0.10 | |
| PBS (pH = 7.4) | 651 nm | 671 nm | 99000 M−1cm−1 | 0.12 | |
| 9 | MeOH | 655 nm | 672 nm | 79900 M−1cm−1 | 0.13 |
| H2O/EtOH (5%) | 653 nm | 671 nm | 73890 M−1cm−1 | 0.10 | |
| PBS (pH = 7.4) | 655 nm | 672 nm | 79900 M−1cm−1 | 0.13 | |
| 10 | MeOH | 654 nm | 672 nm | 63900 M−1cm−1 | 0.14 |
| H2O/EtOH (5%) | 654 nm | 674 nm | 22100 M−1cm−1 | 0.10 | |
| PBS (pH = 7.4) | 654 nm | 669 nm | 39100 M−1cm−1 | 0.09 |
The stokes shifts for all the dyes in methanol, PBS and H2O/EtOH (5%) remain comparable and small (15–20 nm), arising from a small and negligible change in dipole moment between the ground state and the excited state of the SiR fluorophores. With exception of the non-fluorescent amine 6, the quantum yields remain between 13–18% in MeOH and 9–13% in aqueous solution, respectively.
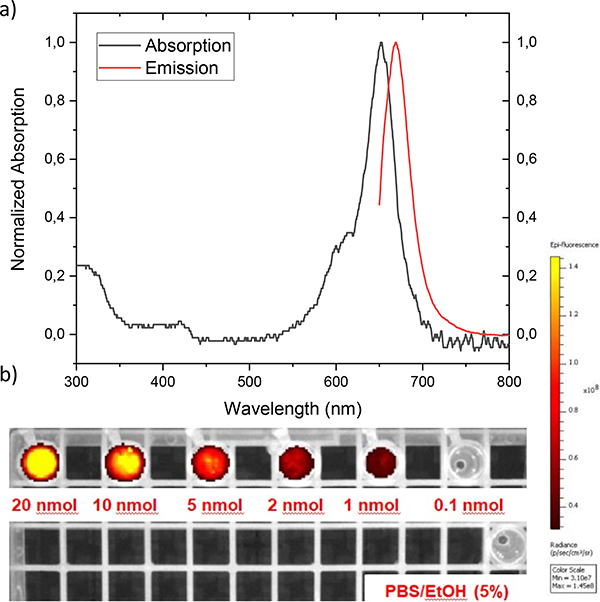
Figure 2a shows the absorption and emission spectra of the rhenium complex 10 in water/ethanol (5%).
Figure 2:
a) Normalized UV/VIS/NIR-absorption and emission spectrum of the rhenium complexed Si-rhodamine in water/ethanol (5%) with an excitation wavelength of 650 nm. b) IVIS-imaging of 10 in PBS/EtOH (5%) with different concentrations to receive the optimal amount of fluorophore for future in vitro/in vivo experiments. The samples were excited with a wavelength of 640 nm and the emission was recorded at a wavelength of 710 nm.
The spectra show a maximum in absorbance at 654 nm and emission at 674 nm (excitation wavelength: 650 nm). Surprisingly, there is no significant change in quantum yield in H2O/EtOH (5%) between the ligand 9 and the rhenium complex 10 (both at ΦF=10%) while the molar absorption coefficient decreases dramatically from 73,890 to 22,100 M−1cm-1. The NIR-dyes 9 and 10 compare well to the FDA approved fluorescent dyes 5-aminolevulinic acid (5-ALA), protoporphyrin IX (PPIX; ΦF=8%) or the NIR-fluorophore indocyanine green (ICG; ΦF=9%) with respect to optical properties and solubility.7
Photobleaching experiments of the synthesized dyes 6, 7, 9 and 10 by irradiation with a wavelength of 650 nm from a xenon lamp in H2O/EtOH (5%) for two hours showed a high photostability of these compounds distinctive for Si-rhodamines and comparable to the photostable cyanine dye Nile Blue (ESI-figure 31).34, 35 The dye 9 shows a very high photostability (4% degradation) whereas SiR 10 has only a decrease of 15% in absorption after two hours irradiation.
We also determined the limit of detection for optical imaging using a conventional, small animal optical imaging scanner. Specifically, we recorded an IVIS-image with different amounts of rhenium complex 10 (figure 2b), indicating that 1 nmol of the probe can be detected without difficulty. This value compares well with the sensitivity of conventional optical fluorophores employed for in vivo applications.36 The stability of complex 10 was assessed in aqueous solution. The rhenium(I) d6-low-spin complex shows robust stabilities in PBS/EtOH (5%) at room temperature and at 37 °C, with no decomplexation observed after 24 hours (ESI table 1). Similar results were observed in challenge experiments with 1 mM histidine in PBS/EtOH (5%) at 37 °C, where complex 10 did not show any decomposition after 24 hours either. The high stability of 10 motivated us to pursue the synthesis for the corresponding 99mTc-radiolabelled analogue.
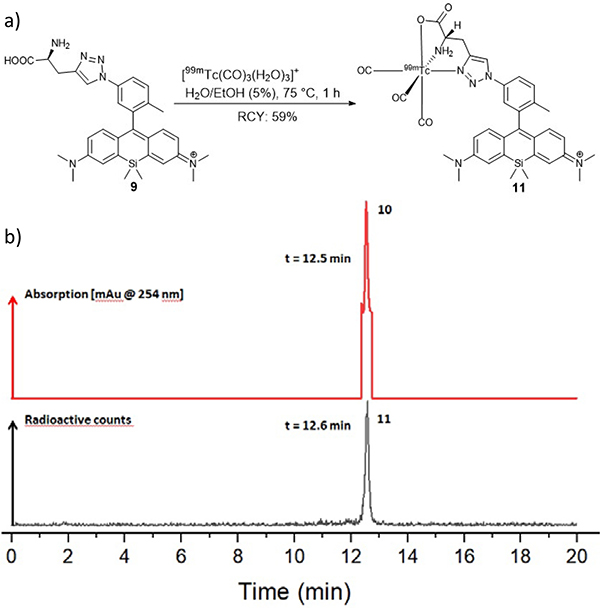
Technetium-99m complexation experiments with ligand 9 were performed under the conditions shown in figure 3a.
Figure 3:
a) Radiolabelling reaction of ligand 9 with a highly reactive [99mTc(CO)3(H2O)3]+-complex in aqueous solution. b) Overview of the HPLC-trace of Re-complex 10 as a non-radioactive surrogate [UV, 254 nm] with a retention time of 12.5 min and the radio-HPLC-trace of the technetium-99m complex 11 [-detector, radioactive counts] with a retention time of 12.6 min. 11 was obtained in a radiochemical yield of 59% with a radiochemical purity of >98% after purification on an analytical HPLC with deionized water and acetonitrile containing 0.1% TFA each.
[99mTc(CO)3(H2O)3]+ was synthesized in accordance with the procedure of Alberto et al.37, 38
The technetium-99m complexed Si-rhodamine 11 was synthesized by incubation of 87 nmol ligand 9 with 81.4 MBq (2.2 mCi) [99mTc(CO)3(H2O)3]+ for 1 hour at 75 °C in an aqueous solution. The desired complex was isolated with a radiochemical yield of 59%, a radiochemical purity greater than 98% and a molar activity of 551 MBq/μmol (3a). The determination of the partition coefficient of 11 with the shake flask assay showed a value for log DpH=7.4= 1.11, indicating that the complex 11 has increased lipophilic character.
The comparison of the retention times of Re-complex 10 (UV-detection at 254 nm, Rt= 12.5 min) and the technetium-99m-complex (decay counts with -detection, Rt= 12.6 min) confirms the identity of the desired final complex 11 (figure 3b).
Complex 11 was isolated using analytical HPLC and was redissolved in PBS/EtOH (5%) to determine the stability in aqueous solution. No additional peaks were observed, indicating good compound stability even after six hours of incubation. A serum stability test at 37 °C by incubation of 11 in human serum plasma showed high stability even after 24 hours analysed by Radio-TLC (ESI table 3).
In conclusion, we here report on the first 99mTc-radiolabelled small-molecule near-infrared Si-rhodamine fluorophore 11. The novel, (radio)metal complexed fluorescent dyes 10 and 11 were successfully characterized. The technetium-99m and rhenium complexed Si-rhodamines can be easily synthesized and show excellent NIR optical properties, are stable under physiological conditions, in PBS, as well as in challenge experiments with histidine and additionally in human serum. Fluorophore 11 has the potential to be further combined with conventionally used, polydisperse 99mTc-nanocolloids for sentinel lymph node (SNL) detection. In a next step the here presented and characterized non-targeted Si-rhodamines 10/11 will be further functionalized for their conjugation to different binding vector molecules to explore their potential of selective enrichment in (tumor) cells and tissues.
Supplementary Material
Acknowledgements
This work was supported by the Wilhelm Sander Stiftung for a grant on bimodal tumor tracers, grant 2018.024.1 (K. K. and C. S. K.) and the NIH, grant R00HL125728 (E. B.). Brett Vaughn is acknowledged for preparation of the technetium-99m-tricarbonyl precursor. Christian Jentschel is acknowledged for scientific support. T. K. acknowledges the FAZIT-Stiftung and the Helmholtz-International Graduate School for Cancer Research for financial support. Martin Schäfer, Dr. Aubry Miller and Dr. Sven Stadlbauer are gratefully thanked for helpful scientific discussions. Heidelberg University and Prof. Peter Comba are thanked for providing IR- and NMR-measurements.
Footnotes
Conflicts of interest
The authors have no conflicts to declare.
References
- 1.Escobedo JO, Rusin O, Lim S and Strongin RM, Curr Opin Chem Biol, 2010, 14, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belov VN, Wurm CA, Boyarskiy VP, Jakobs S and Hell SW, Angew Chem Int Ed Engl, 2010, 49, 3520–3523. [DOI] [PubMed] [Google Scholar]
- 3.Zhu S, Yang Q, Antaris AL, Yue J, Ma Z, Wang H, Huang W, Wan H, Wang J, Diao S, Zhang B, Li X, Zhong Y, Yu K, Hong G, Luo J, Liang Y and Dai H, Proc Natl Acad Sci U S A, 2017, 114, 962–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antaris AL, Chen H, Diao S, Ma Z, Zhang Z, Zhu S, Wang J, Lozano AX, Fan Q, Chew L, Zhu M, Cheng K, Hong X, Dai H and Cheng Z, Nat Commun, 2017, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeno T, Nagano T and Hanaoka K, Chem Asian J, 2017, 12, 1435–1446. [DOI] [PubMed] [Google Scholar]
- 6.Luo S, Zhang E, Su Y, Cheng T and Shi C, Biomaterials, 2011, 32, 7127–7138. [DOI] [PubMed] [Google Scholar]
- 7.Zhang DY, Singhal S and Lee JYK, Neurosurgery, 2019, 85, 312–324. [DOI] [PubMed] [Google Scholar]
- 8.Weissleder R, Nat Biotechnol, 2001, 19, 316–317. [DOI] [PubMed] [Google Scholar]
- 9.Ando N, Soutome H and Yamaguchi S, Chem Sci, 2019, 10, 7816–7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godard A, Kalot G, Pliquett J, Busser B, Le Guevel X, Wegner KD, Resch-Genger U, Rousselin Y, Coll JL, Denat F, Bodio E, Goze C and Sancey L, Bioconjug Chem, 2020, 31, 1088–1092. [DOI] [PubMed] [Google Scholar]
- 11.Bodio E, Denat F and Goze C, J Porphyr Phthalocya, 2020, 23, 1159–1183. [Google Scholar]
- 12.Koide Y, Urano Y, Hanaoka K, Piao W, Kusakabe M, Saito N, Terai T, Okabe T and Nagano T, J Am Chem Soc, 2012, 134, 5029–5031. [DOI] [PubMed] [Google Scholar]
- 13.Koide Y, Urano Y, Hanaoka K, Terai T and Nagano T, ACS Chem Biol, 2011, 6, 600–608. [DOI] [PubMed] [Google Scholar]
- 14.Weissleder R and Ntziachristos V, Nat Med, 2003, 9, 123–128. [DOI] [PubMed] [Google Scholar]
- 15.Culver J, Akers W and Achilefu S, J Nucl Med, 2008, 49, 169–172. [DOI] [PubMed] [Google Scholar]
- 16.van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, de Jong JS, Arts HJ, van der Zee AG, Bart J, Low PS and Ntziachristos V, Nat Med, 2011, 17, 1315–1319. [DOI] [PubMed] [Google Scholar]
- 17.Lu Z, Pham TT, Rajkumar V, Yu Z, Pedley RB, Arstad E, Maher J and Yan R, J Med Chem, 2018, 61, 1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seibold U, Wangler B, Schirrmacher R and Wangler C, Biomed Res Int, 2014, 2014, 153741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Lin TP, Liu S, Huang CW, Hudnall TW, Gabbai FP and Conti PS, Chem Commun (Camb), 2011, 47, 9324–9326. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Li D, Zhang Z, Surya Prakash GK, Conti PS and Li Z, Chem Commun (Camb), 2014, 50, 7371–7373. [DOI] [PubMed] [Google Scholar]
- 21.Schottelius M, Wirtz M, Eiber M, Maurer T and Wester HJ, EJNMMI Res, 2015, 5, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robu S, Schottelius M, Eiber M, Maurer T, Gschwend J, Schwaiger M and Wester HJ, J Nucl Med, 2017, 58, 235–242. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee SR, Pullambhatla M, Byun Y, Nimmagadda S, Foss CA, Green G, Fox JJ, Lupold SE, Mease RC and Pomper MG, Angew Chem Int Ed Engl, 2011, 50, 9167–9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mindt TL, Struthers H, Brans L, Anguelov T, Schweinsberg C, Maes V, Tourwe D and Schibli R, J Am Chem Soc, 2006, 128, 15096–15097. [DOI] [PubMed] [Google Scholar]
- 25.Liu S and Edwards DS, Chem Rev, 1999, 99, 2235–2268. [DOI] [PubMed] [Google Scholar]
- 26.Lukinavicius G, Umezawa K, Olivier N, Honigmann A, Yang G, Plass T, Mueller V, Reymond L, Correa IR Jr., Luo ZG, Schultz C, Lemke EA, Heppenstall P, Eggeling C, Manley S and Johnsson K, Nat Chem, 2013, 5, 132–139. [DOI] [PubMed] [Google Scholar]
- 27.Bertozzi CR and Shieh P, US Patent US9410958B2, 2015. [Google Scholar]
- 28.Shieh P, Siegrist MS, Cullen AJ and Bertozzi CR, Proc Natl Acad Sci U S A, 2014, 111, 5456–5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Struthers H, Spingler B, Mindt TL and Schibli R, Chem Eur J, 2008, 14, 6173–6183. [DOI] [PubMed] [Google Scholar]
- 30.Darab JG and Smith PA, Chemistry of Materials, 1996, 8, 1004–1021. [Google Scholar]
- 31.Davies LH, Kasten BB, Benny PD, Arrowsmith RL, Ge H, Pascu SI, Botchway SW, Clegg W, Harrington RW and Higham LJ, Chem Commun (Camb), 2014, 50, 15503–15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langdon-Jones EE, Symonds NO, Yates SE, Hayes AJ, Lloyd D, Williams R, Coles SJ, Horton PN and Pope SJ, Inorg Chem, 2014, 53, 3788–3797. [DOI] [PubMed] [Google Scholar]
- 33.Turnbull WL, Yu L, Murrell E, Milne M, Charron CL and Luyt LG, Org Biomol Chem, 2019, 17, 598–608. [DOI] [PubMed] [Google Scholar]
- 34.Koide Y, Urano Y, Hanaoka K, Terai T and Nagano T, J Am Chem Soc, 2011, 133, 5680–5682. [DOI] [PubMed] [Google Scholar]
- 35.Martinez V and Henary M, Chemistry, 2016, 22, 13764–13782. [DOI] [PubMed] [Google Scholar]
- 36.Hameed S, Chen H, Irfan M, Bajwa SZ, Khan WS, Baig SM and Dai Z, Bioconjug Chem, 2019, 30, 13–28. [DOI] [PubMed] [Google Scholar]
- 37.Alberto R, Ortner K, Wheatley N, Schibli R and Schubiger AP, J Am Chem Soc, 2001, 123, 3135–3136. [DOI] [PubMed] [Google Scholar]
- 38.Konkankit CC, Vaughn BA, MacMillan SN, Boros E and Wilson JJ, Inorg Chem, 2019, 58, 3895–3909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.